Epilepsy & Seizures
Ambiguous paroxysmal events
Mar. 22, 2024
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
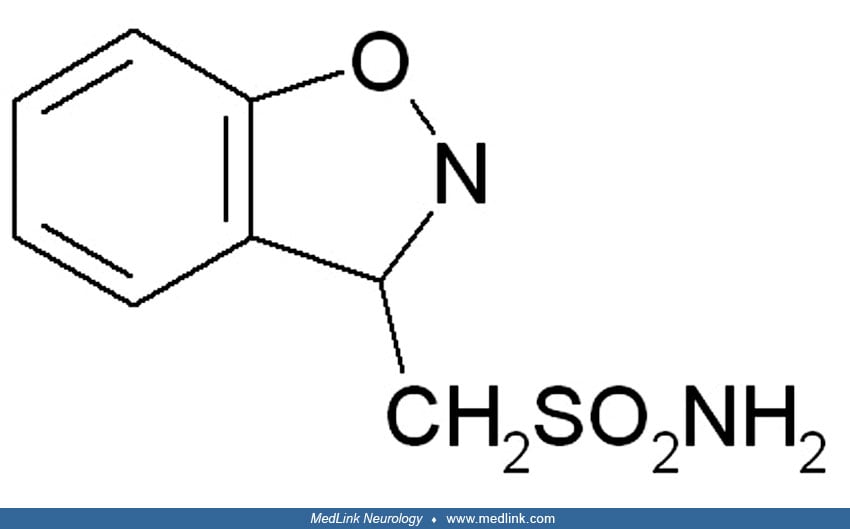
Zonisamide is chemically classified as a sulfonamide and is unrelated to other antiepileptic drugs. Originally developed in Japan for the treatment of epilepsy, it was later approved in 1989, and has since been licensed in many countries. It was under investigation for cerebral ischemia, but no development has been reported for this indication since 1994. Zonisamide was approved by the Food and Drug Administration in 2000 for marketing as an add-on adult focal epilepsy treatment. It is now approved in Europe as monotherapy for the treatment of partial seizures in adults affected by newly diagnosed epilepsy.
Pharmacodynamics. The mechanism of the antiepileptic action of zonisamide remains unclear, but likely mechanisms include:
• Blockade of sodium channels. |
Zonisamide does not potentiate the synaptic activity of gamma-aminobutyric acid. In experimental animals, zonisamide suppresses the tonic-extensor components of maximal electroshock seizures and prevents the propagation of seizures from the cortex to the subcortical structures evoked by cortical freezing. Electroencephalographic studies on animal models of epilepsy show that zonisamide, like phenytoin and carbamazepine, restricts the spread or propagation of seizures and, like sodium valproate, suppresses the epileptogenic focus activity. Neuroprotective effect of zonisamide has been demonstrated in the rat model of focal ischemia induced by a transient occlusion of the left middle cerebral artery. One explanation of this neuroprotective effect is by scavenging of free radicals. Another explanation is that zonisamide is effective in reducing neuronal damage by a mechanism involving decreased ischemia-induced extracellular glutamate accumulation and interruption of excitotoxic pathways (14). As therapeutic concentrations of zonisamide positively modulate recombinant and native glycine receptors, anticonvulsant effects of zonisamide may be mediated via this action (05).
Pharmacokinetics. Pharmacokinetic profile of zonisamide in humans is as follows:
• Following a 200 to 400 mg oral zonisamide dose, peak plasma concentrations (2 to 5 mg/mL) occur within 2 to 6 hours. The dose-serum level correlation is linear up. Almost 100% of it is absorbed. A steady state is reached in about 2 weeks. | |
• Approximately 40% of zonisamide is bound to plasma proteins, primarily albumin. | |
• Zonisamide has saturable binding to red blood cells (RBCs), especially to intracellular carbonic anhydrase, and is found in higher concentrations in RBCs than in plasma. | |
• Zonisamide is excreted mainly in urine, and the renal clearance is increased by enzyme-inducing antiepileptic drugs. In adults, the elimination half-life of zonisamide is 50 to 62 hours. | |
• Following an oral administration of 14-C-zonisamide to healthy volunteers, only zonisamide is detected in the plasma. Of the excreted dose, 35% is the parent drug, 15% the metabolite sulphamoylacetyl phenol, and 50% a glycuronide of this metabolite. Reduction of zonisamide to sulphamoylacetyl phenol is mediated by cytochrome P450 isoenzyme 3A4 (CYP3A4). Pharmacokinetics of zonisamide after oral administration is like a linear first-order elimination 2-compartment model, which may provide a reference for clinical use of zonisamide in Chinese adults (25). | |
• The change in zonisamide serum concentrations in a pregnant woman suggests an increase in clearance at the end of the second trimester (23). | |
• A simple and accurate liquid chromatography/mass spectrometry method for quantification of zonisamide in human plasma has been developed and validated over the concentration range of 0.5 to 50 μg/mL (18). |
Routes of administration. Zonisamide is marketed as tablets for oral use. A liquid formulation for intravenous use is available. Preclinical studies of intranasal zonisamide show that drug targeting efficiency and direct transport percentage into the brain are 149.54% and 33.13%, respectively, indicating that a significant fraction of zonisamide is transported directly from the nose to the brain (09). Zonisamide has a low absolute bioavailability (54.95%) but the highest value of the ratio between the area under the curve (AUC) between brain and plasma, suggesting lower systemic adverse events and noninferior effects on central nervous system compared to intravenous and oral routes.
A clinical retrospective study of zonisamide as adjunctive therapy in children with epilepsy that was not responding to other antiepileptic drugs found that it was an effective and safe treatment (16).
A randomized, multicenter trial has compared low-dose (3 to 4 mg/kg/day) or high-dose zonisamide (6 to 8 mg/kg/day) in childhood epilepsy (08). Lower doses of zonisamide had a similar efficacy and more beneficial neurocognitive effects compared to higher doses, which interfere with language development.
Four key phase III clinical trials as well as subsequent postmarketing trials that have expanded the therapeutic indication for zonisamide are reviewed in detail in a publication (11). Clinical data support zonisamide as a viable first-line and adjunctive therapy for partial-onset epilepsy.
Results of a phase III, randomized, double-blind, parallel-group, clinical trial showed that zonisamide was non-inferior to controlled-release carbamazepine and could be useful as an initial monotherapy for patients newly diagnosed with partial epilepsy (01).
A systematic review of clinical trials showed that zonisamide is effective as an add-on treatment in patients with focal epilepsy uncontrolled by one or more concomitant antiepileptic drugs, and there was moderate-quality evidence that zonisamide was more successful than placebo at reducing the frequency of seizures by at least 50% (02).
Zonisamide is indicated for the treatment of partial seizures in adults with epilepsy.
West syndrome. Zonisamide has been used with some success in patients who did not respond to pyridoxine or valproic acid.
Absence epilepsy. A post-marketing study has shown that add-on zonisamide is efficient and well tolerated in juvenile patients with refractory absence epilepsy (31).
Infantile spasms. Zonisamide treatment has been shown to be beneficial in some cases of infantile spasms.
Tremor. Zonisamide reduces tremor amplitude and has been used for controlling essential tremor involving the head (29). A systematic review of randomized controlled trials concluded that there is insufficient evidence to assess the efficacy and safety of zonisamide as treatment for essential tremor (03).
Parkinson disease. Activation of dopamine synthesis and the moderate level of monoamine oxidase B inhibition are the main mechanisms of zonisamide effects on Parkinson disease. Zonisamide may be effective in reducing the duration of “off” time in patients with Parkinson disease treated with levodopa. Zonisamide has additional targets, including neuroinflammation and the voltage-gated sodium channel Nav1.6, which may contribute to its reported neuroprotective role Parkinson disease (12). In a phase 3 clinical trial on patients with Parkinson disease and Lewy body dementia, daily administration of 25 or 50 mg zonisamide significantly improved motor function compared with placebo; both doses were safe and well tolerated (21). Two phase 2 and 3 randomized trials on patients with Parkinson disease and Lewy body dementia recently reported a statistically significant improvement in motor function in those receiving zonisamide as an adjunctive treatment to levodopa (24).
Obesity. Weight loss was originally noted as an adverse effect of zonisamide but has been investigated for therapeutic use in obesity.
Migraine. Zonisamide has been used for preventive treatment of chronic migraine in patients who are refractory or intolerant to topiramate (32). In another study on refractory migraine patients, zonisamide therapy did not result in a statistically significant beneficial effect on headache or on associated symptoms.
Zonisamide is an effective prophylactic treatment for patients with chronic and episodic cluster headache disorders (17).
Brain tumor-related epilepsy. Preliminary results of an open study of zonisamide as add-on in a patient population indicate that it may represent a valid alternative to other add-on antiepileptic drugs (19).
Secondary paroxysmal dystonia (06).
Alcohol dependence. In an open-label study, zonisamide was found to be well tolerated and associated with improvement in alcohol craving (28).
Tardive dyskinesia. Results of an open-label study indicate that zonisamide may be safe and effective for the treatment of tardive dyskinesia associated with antipsychotic treatment (13).
Catatonia. Refractory periodic catatonia in a patient with schizophrenia was successfully treated with a combination of zonisamide and aripiprazole -- a partial agonist of dopamine D2 receptor (22).
Bulimia nervosa. In an open-label study, zonisamide was effective in bulimia nervosa but was associated with a high discontinuation rate (10).
Obstructive sleep apnea. In a randomized study, zonisamide was shown to reduce obstructive sleep apnea by mechanisms related to carbonic anhydrase inhibition (07).
Bipolar disorder. Zonisamide may be useful as an adjunctive therapy for the management of acute phases and weight gain in bipolar disorder (04).
Neuropathic pain. Zonisamide has shown efficacy in patients with neuropathic pain.
Zonisamide is contraindicated in patients who have demonstrated hypersensitivity to sulfonamides or zonisamide.
Zonisamide is added when the older established antiepileptic drugs fail to control the seizures. The dose is increased until the seizure control is reached. High doses of zonisamide are effective and safe in pharmacoresistant epileptic patients, but therapeutic drug monitoring is recommended (20). Long-term maintenance therapy is possible if no serious adverse reactions occur. The long-term effectiveness of zonisamide in West syndrome has been shown in follow-up from 24 to 79 months (30). Zonisamide, as monotherapy or adjunctive therapy, has been demonstrated to be safe, effective, and well tolerated as a long-term treatment option in patients with various seizure types. Zonisamide has a good safety profile and sustained efficacy in the long-term adjunctive treatment of refractory partial epilepsy.
Zonisamide is available in capsules of 25, 50, and 100 mg, and the daily dose range is 100 to 600 mg.
Anesthesia. No special precautions.
Pregnancy. Women of childbearing age who are given zonisamide should be advised to use effective contraception. Zonisamide was teratogenic in mice, rats, and dogs and embryolethal in monkeys when administered during the period of organogenesis. These findings suggest that the use of zonisamide during pregnancy in humans may present a significant risk to the fetus. Zonisamide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Zonisamide serum concentrations may fall by more than 40% during pregnancy, which may worsen seizure control, and therapeutic drug monitoring with dose adjustments are useful measures to cope with this (26). Zonisamide concentration in breast milk is the same as that in maternal plasma, but serum levels in neonates decrease during the first month of life during nursing. Alternative drugs are preferred, but if zonisamide must be given, the infant should be monitored for drowsiness, adequate weight gain, and developmental milestones.
Pediatric. Use of zonisamide is safe in children less than 16 years of age, and the adverse event profile is like that of adults in studies in the United States and Europe.
An extension study of a phase 3 trial showed that approximately one-third of children treated with zonisamide had 5% or greater weight loss, but it was not associated with any effects on growth and development (15).
Geriatric. Single-dose pharmacokinetic parameters are similar in healthy volunteers of all ages. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of the decreased.
However, data from uncontrolled studies suggest that zonisamide is effective and well tolerated when administered as monotherapy or adjunctive antiepileptic treatment in the elderly (27).
Concurrent medication with drugs that either induce or inhibit CYP3A4 may alter serum concentrations of zonisamide. Concomitant administration of phenytoin and carbamazepine increases zonisamide plasma clearance and the half-life of zonisamide is decreased to 27 hours by phenytoin, to 38 hours by phenobarbital and carbamazepine, and to 46 hours by valproic acid. Plasma protein binding of phenytoin and carbamazepine is not affected by zonisamide administration. When zonisamide is administered concomitantly with phenytoin, phenobarbital, or carbamazepine, it is necessary to monitor the plasma concentration of zonisamide to adjust its dosage. However, zonisamide has a favorable interaction profile in relation to other drugs, with less clinically significant drug interactions than older antiepileptic drugs.
Zonisamide has not been associated with elevations in serum aminotransferase levels, and clinically apparent drug-induced liver disease has been reported with its use but is very rare. Frequent adverse events (ataxia, somnolence, agitation, irritability, and anorexia) were significantly associated with zonisamide in clinical trials. Other reported adverse effects are:
Toxic epidermal necrolysis. A case of Stevens-Johnson syndrome/ toxic epidermal necrolysis associated with zonisamide was successfully treated with IVIG and the drug was replaced with levetiracetam for seizure control (33).
Kidney stones. The available data suggest that the risk of developing renal calculi during zonisamide treatment is low.
Oligohidrosis and hyperthermia in children. Zonisamide may cause decreased sweating, which may be accompanied by elevated body temperature, especially in the summer. Body temperature should be carefully monitored and recovery from hyperthermia should follow discontinuation of zonisamide.
Neuropsychiatric adverse events. The most significant of these are depression, psychomotor slowing and difficulty with concentration, and psychosis. Visual hallucinations have been associated with zonisamide. The risk of psychotic episodes is higher in younger patients. Although the relation of psychotic episodes to zonisamide therapy cannot be proven, particularly in patients with polytherapy, it is recommended that zonisamide be discontinued if this adverse reaction appears.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

K K Jain MD†
Dr. Jain was a consultant in neurology and had no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Epilepsy & Seizures
Mar. 22, 2024
Epilepsy & Seizures
Mar. 07, 2024
Epilepsy & Seizures
Mar. 06, 2024
Movement Disorders
Mar. 06, 2024
Epilepsy & Seizures
Mar. 05, 2024
Epilepsy & Seizures
Mar. 05, 2024
Epilepsy & Seizures
Mar. 04, 2024
Epilepsy & Seizures
Feb. 29, 2024