Peripheral Neuropathies
Thallium neuropathy
Jul. 17, 2024
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
The clinical article reviews the clinical presentation, diagnosis, pathogenesis, and management of Bell palsy. Bell palsy is an acute, usually unilateral mononeuropathy of the seventh cranial nerve. The disorder is presumed to be inflammatory and associated with one of several different viral etiologies, usually herpes simplex virus-1. The disorder is usually self-limiting, with up to 90% of affected subjects showing full recovery. Recovery occurs within 6 months, and most will improve within 3 weeks of onset of the disorder. The differential diagnosis is outlined in this clinical article, and specific tests may be indicated for certain patients depending on the clinical evaluation. Early treatment with steroids is recommended. Several large randomized studies clearly show that prednisone (prednisolone), given within 48 hours of onset of the palsy, improves recovery rate and promotes early recovery in affected patients. Combination therapy with antivirals is not recommended based on current clinical trial and meta-analysis data. In addition, supportive care and eye care are critical.
Bell palsy was named for Sir Charles Bell (1774-1842), a British neurologist, who in 1821 described the condition and demonstrated that there was a separation of motor and sensory innervation in the face. Similar descriptions of the disorder were, however, previously published by Nikolaus A Friedreich (1825-1882) in 1798 and Richard Powell in 1813.
Bell palsy is usually acute in onset, unilateral, and there is no evidence of disease of the central nervous system, ear, or posterior fossa. Approximately 60% of patients have a preceding viral illness. Numbness or pain in front or behind the ear is present in about 50% of patients. Only 6.5% of patients will have recurrent facial palsy (14). In this meta-analysis of 222 subjects, sidedness of recurrent disease did not affect patient prognosis, but recovery rate was lower in patients with recurrent compared to primary Bell palsy. The majority of patients (90%) will have a decreased ipsilateral stapes reflex, approximately 25% have impaired taste perception on direct questioning, and in 10% there is loss or significant decrease of ipsilateral tearing or submandibular salivary flow (02). The history should include a careful assessment of the following: date of onset, rapid versus slow onset, partial versus complete palsy, recurrence, same side versus alternating, recent accident or blow to the head, recent viral infections, pain behind the ear, change in auditory acuity, vertigo, and change in taste; if pregnant, detailed questions about function of the affected eye, past and present history of malignancies, HIV and Lyme disease status, recent immunizations (eg, influenza, polio, or rabies), use of medications like isoniazid, and a family history of facial palsy.
On examination, the features of Bell palsy include impairment of voluntary movement of facial and platysmal muscles, facial muscles pulled to the opposite side on smiling, saliva and food collected on the paralyzed side, and on attempting to close the eye, the eyeball is diverted upward and slightly outwards to avoid corneal exposure (Bell phenomenon). With a lesion proximal to the geniculate ganglion, there may be decreased tearing in the affected eye. When the chorda tympani is affected there may be a decrease in salivation and taste in the anterior two thirds of the tongue. The House-Brackmann facial grading system allows a systematic grading of facial weakness in Bell palsy (25). The scale can be applied using an automated system that uses artificial intelligence to achieve 100% accuracy (35).
Description | Characteristics |
Normal | Normal facial function in all areas |
Mild dysfunction | Slight weakness noticeable on close inspection; may have very slight synkinesis |
Moderate dysfunction | Obvious, but not disfiguring, difference between two sides; noticeable, but not severe, synkinesis, contracture, or hemifacial spasm; complete eye closure with effort |
Moderately severe dysfunction | Obvious weakness or disfiguring asymmetry; normal symmetry and tone at rest; incomplete eye closure |
Severe dysfunction | Only barely perceptible motion; asymmetry at rest |
Total paralysis | No movement |
In order to exclude other disorders that mimic Bell palsy, the patient should be examined for masses in the head or neck, signs of ear vesicles or infection, and facial twitching.
Late complications of Bell palsy usually do not occur until 3 to 4 months after the onset of the paralysis. The most common late complication is contracture, which may be commonly accompanied by synkinesis in approximately 16% of subjects (43). Both complications are directly proportional to the degree of denervation and the rate of recovery (02). Ptosis of the eyebrow may require surgical correction if severe. The last late complication to develop is gustatory (crocodile) tearing in which there is aberrant regeneration of secretory fibers to the lacrimal glands resulting in tearing during eating. Gustatory tearing usually does not occur until 4 months after the onset of Bell palsy.
Full recovery occurs in approximately 80% to 90% of patients, and 10% may have a recurrence of Bell palsy. Recovery usually occurs in 4 to 6 months and at most by 12 months (39; 37). A facial motor potential amplitude of less than 10% of normal is an indicator of poor prognosis, however, even in this group 30% to 50% of patients will have complete recovery (48). Other indicators of a poor recovery include age over 60 years, untreated hypertension, non-ear pain, abnormal electrogustometry at onset, and submandibular flow rate of less than 45% (30; 13).
A 64-year-old man developed acute left facial weakness soon after waking in the morning. He had difficulty moving food from the left side of his mouth and observed that he could not completely close his left eyelid. Concomitant with the weakness, he developed a dull tenderness behind his left ear. He had no specific sensory symptoms, no diplopia, and no associated weakness of his right face or limbs. There was no specific viral illness or vesicular rash associated with or preceding his current illness.
He had coronary artery bypass surgery for unstable angina 5 years previously as well as a history of controlled hypertension. At the time of surgery, epicardial pacemaker leads were left in place, precluding subsequent magnetic resonance imaging. Medications included isosorbide dinitrate, prazosin, diltiazem XR, and 325 mg aspirin per day. There was no previous history of facial weakness, stroke, HIV, Lyme disease exposure, or diabetes. He was a previous heavy smoker and had stopped drinking heavily 30 years earlier. There was no family history of any neurologic disease including facial weakness.
The patient's general examination was unremarkable and vital signs were normal. There was no intra-aural vesicular rash and the otoscopic examination was normal. The patient had flattening of the left nasolabial fold, and partial paralysis of all facial innervated muscles on the left side, with a positive Bell phenomenon on the left, and an impaired ipsilateral corneal blink response, but intact consensual response.
Left eye closure was only moderately compromised and the patient had no corneal drying or abrasions. The patient had no left hyperacusis, and taste was normal. Extraocular movements, pupillary responses, ophthalmoscopic examination, facial sensation, and bulbar function were all unremarkable. There was bilateral high-frequency hearing impairment, worse on the nonparalyzed side. The remaining neurologic examination was unremarkable.
Electrophysiological studies performed 1 week after the onset of the facial weakness were abnormal. Compared to the unaffected side, there was a 40% decrease in the left direct facial compound action muscle potential amplitude and a 20% increase in the distal latency.
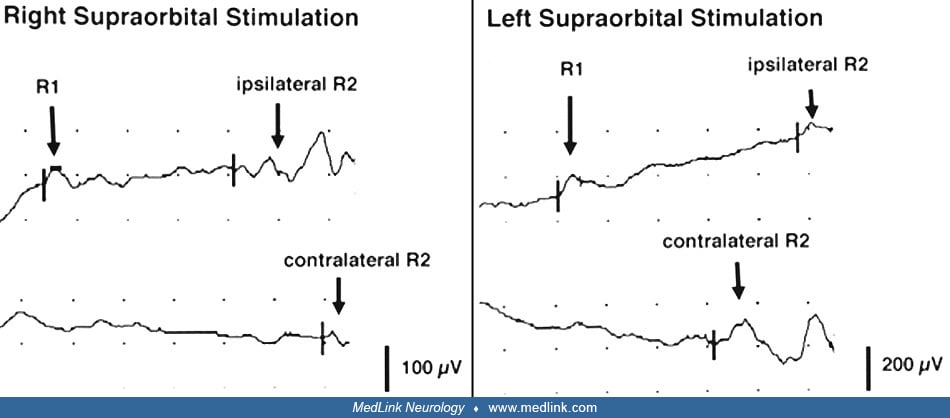
With left-sided supraorbital stimulation, the ipsilateral R1 was delayed with prolongation of the ipsilateral R2 but a normal contralateral R2. With right-sided supraorbital stimulation, the ipsilateral R1 and R2 were normal, but the contralateral was delayed.
A subsequent needle electromyography examination revealed evidence of acute denervation in left-sided facial innervated muscles. The complete blood count, serum electrolytes and creatinine, fasting and 2-hour postprandial blood glucose, glycosylated hemoglobin, erythrocyte sedimentation rate, antinuclear antibody test, rheumatoid factor test, antineutrophil cytoplasmic autoantibody, Lyme titers, acute Epstein-Barr virus titers, and varicella-zoster virus titers were normal or negative. Herpes simplex virus-1 IgM titers were normal and IgG titers were mildly elevated. The chest x-ray showed no evidence of a pulmonary nodule and the head CT with contrast showed no evidence of a mass or infiltration along the course of the intracranial left facial nerve.
The patient took 60 mg of prednisone for 5 days and then rapidly tapered the medication over the next 5 days. Prednisone therapy was commenced within 24 hours of the onset of the facial weakness. The patient was prescribed eye ointment, and left corneal function was carefully monitored. The patient fully recovered facial function after 3 months.
Bell palsy is a mononeuropathy of the seventh cranial nerve of sudden onset causing a lower motor neuron type of facial weakness. Evidence suggests the main etiologic agent is a herpes simplex virus-1 infection of the nerve (01).
Although the pathogenesis and pathophysiology of Bell palsy are unclear, studies report an association of acute facial palsy with a variety of viruses including herpes simplex virus-1, varicella-zoster virus, mononucleosis, cytomegalovirus, adenovirus, influenza virus, Coxsackie, polio, mumps (33), HIV (24), and West Nile virus (60). Herpes simplex viruses may be the most likely candidates. Polymerase chain reaction of endoneurial or tear fluid has isolated herpes simplex virus-1 genomes in up to 86%, and varicella-zoster virus in 43% of geniculate ganglion preparations from patients with Bell palsy compared to controls, but not in muscle biopsies from the same subjects (36). Reactivation of herpes simplex virus-1 probably results in the initial facial weakness, and the virus becomes undetectable with recovery (01; 31). Unlike herpes simplex virus-1, influenza vaccination, and specifically monovalent pandemic influenza vaccine, does not appear to be associated with Bell palsy. The incidence rate of Bell palsy in a primary health care database, THIN, in the United Kingdom was 38.7 per 100,000 person-years. Both acute respiratory infection consultations and pregnancy were found to be confounders. When adjusted for seasonality, acute respiratory infection consultations, and pregnancies, the relative incidence during the 42 days after vaccination with an influenza vaccine was 0.85 (95% CI: 0.72-1.01) (62).
Bell palsy may possibly have an immune etiology, suggested by its association with interferon-alpha2b therapy (41; 26). Histological studies of the facial nerve during the acute stage of Bell palsy, either at autopsy or during surgery, have shown signs of edema, perivascular perineurial lymphocytic and macrophage infiltration of the nerve, an increase in the axon to myelin surface ratio (thinning of myelin), and a decrease in the total fiber count (46). Bell palsy has also been associated with smallpox vaccination (51). Interestingly, consistent with the hyperimmune hypothesis, Bell palsy is infrequently observed in patients with COVID-19, although this is controversial (10). The risk of Bell palsy was increased (OR 2·385 for CoronaVac and 1·755 for BNT162b2) within 42 days of COVID vaccination. Vaccinated cases were matched (1:4) with nonvaccinated controls (61).
Bell palsy has a heritability estimate of 4% to 14%, suggesting a genetic component in the etiology and a possible autosomal dominant inheritance associated with rs9357446-A (55).
The incidence of Bell palsy is 20 to 30 per 100,000 persons (02; 30; 12). The rate increases with age up to the fourth decade and then remains steady until the eighth decade when it again increases. Bell palsy is seen with almost equal frequency (ratio 46:54) in men and women (12). Onset of facial paralysis almost equally occurs on both sides of the face (right slightly greater than left) (47; 13), and approximately 5% of adult patients (47) and 6% of children (16) will have a recurrent palsy affecting the same or opposite side (47). Preeclampsia, diabetes, and hypertension have all been associated with an increased incidence of Bell palsy. The incidence of Bell palsy is elevated in the late phases of pregnancy. Late-term pregnancy-associated Bell palsy (PABP) is associated with worse long-term outcomes in Bell palsy (44). In patients who received early corticosteroid therapy, eFACE scores were worse in those with pregnancy-associated Bell palsy compared to nonpregnancy-associated cases. A trend toward improved outcomes was demonstrated within both groups for those treated with corticosteroids compared to those who were not.
The differential diagnosis of acute facial weakness that may mimic Bell palsy is encyclopedic. Acute trauma to the facial nerve should always be considered and excluded. The following are some of the more common or treatable conditions that may mimic Bell palsy:
Neoplasms of the facial nerve.
• Benign tumors. Schwannomas of the facial nerve can present with recurrent acute facial palsy (45). More rarely neurofibromas, glomus jugulare tumors, and hemangiomas may compress the facial nerve (45).
• Malignant tumors. Parotid malignancies, acute lymphoblastic leukemia, basal cell carcinoma, squamous cell carcinoma from the middle or external ear canal, adenocarcinoma of the cerumen glands, and temporal bone metastases from the breasts, lungs, and kidneys may also cause facial nerve paralysis (45).
Infections of the middle ear, or mastoid (45).
Hemifacial spasm. Most series have not found an association between Bell palsy and hemifacial spasm, although synkinesis of facial muscles may occur in both conditions (46).
Demyelinating disorders. Including multiple sclerosis and acute inflammatory demyelinating polyneuropathy.
Cerebellopontine angle tumors or aneurysms. Acoustic schwannomas, meningiomas, and glomus jugulare tumors may compress the seventh cranial nerve at the cerebellopontine angle.
Lyme disease. Facial nerve paralysis is the most common neurologic complication of Lyme disease. There may be a history of a tick bite, rash, or arthritis (39; 09).
Neurosarcoidosis. Association of seventh nerve palsy with uveitis, parotid gland enlargement, and fever (Heerfordt syndrome).
Diabetes mellitus. Diabetes mellitus is an unusual cause of peripheral seventh nerve palsy. However, 20% of 288 patients in one large series of Bell palsy were found to be diabetic (50). Generally, the prognosis for recovery is worse in diabetic compared to nondiabetic patients. In one study, the recovery rate in diabetics was 52.6%, which was much lower than that in the nondiabetic group (82.5%) at 6 months after onset (28). However, a study of 60 patients with Bell palsy concluded that diabetes does not affect the severity, recovery rate from, or healing of Bell palsy. Participants were followed for 1 to 3 years and treated with 1 mg/kg prednisone/day for 5 days followed by a tapered dose reduction and acyclovir 200 mg orally every 4 hours, five times daily, for 5 days (52).
Syphilis and tuberculosis. Both are rare but treatable causes of facial nerve paralysis. Syphilitic gumma compress the nerve within the temporal bone, whereas tuberculosis of the middle ear causes inflammation of the tympanic segment.
Melkersson-Rosenthal syndrome. Alternating seventh cranial nerve palsy occurs mainly in children. It is associated with a large-fissured tongue and swelling of the face and lips (42).
Ramsay Hunt syndrome. Ramsay Hunt syndrome is Varicella-zoster virus infection of the geniculate ganglion associated with facial weakness and a painful vesicular eruption of the external ear canal.
Immune-checkpoint inhibitors (ICIs). Patients with melanoma treated with immune-checkpoint inhibitors can develop unilateral Bell palsy (05). Median time-to-onset of Bell palsy from immune-checkpoint inhibitors initiation was 15 weeks. All the patients completely recovered from Bell palsy with prednisone alone, or prednisone plus valaciclovir.
The most important aspect of the diagnostic workup is the evaluation of the history and physical examination outlined under clinical manifestations. In addition, electromyography and MRI imaging of the facial nerve may offer important prognostic information, as well as help to exclude other less benign diagnoses. Radiologic tests and special laboratory tests as outlined below are indicated in certain patients based on clinical suspicion but are not cost-effective in all patients with suspected Bell palsy.
Electrophysiological studies. Electroneurography of the direct facial response and the blink reflex performed 1 week after the onset of Bell palsy is helpful in confirming the presence of a peripheral facial neuropathy (15). In the first 1 to 2 weeks of facial weakness, proximal conduction block may be present, but the nerve distal to the fallopian canal can still be stimulated and lags behind the degree of clinical deficit. Maximum Wallerian degeneration occurs by 7 days, although the electroneurographic response may decrease for up to 10 to 14 days. Up to 50% of patients with a poor electrophysiological prognosis (facial motor potential is 10% or less of normal) eventually show a complete recovery of function (48); thus, electrophysiology should serve as a useful adjunct to sound clinical judgment. In a population-based study from Ontario, Canada, 224 patients were surveyed, of whom 91 underwent formal neurologic assessment. Of these, 44 were studied electrophysiologically, and 32 fulfilled the clinical criteria for Bell palsy. Blink responses were the most useful test, showing diagnostic sensitivity of 81% and specificity of 94% compared to the contralateral control side. In contrast, facial EMG was not useful (22). Transcranial magnetic stimulation allows study of the intracranial facial nerve and in the future may prove useful in determining early prognosis in Bell palsy (49).
Radiographic imaging.
• MRI with and without gadolinium: contrast enhancement of the facial nerve, especially the meatal portion, may last for several months, but has not proved of prognostic value (27). Imaging to rule out neoplasms or alternative diagnoses should be considered in patients with no improvement or progressive weakness (11). | |
• CT with and without contrast of the brainstem, cerebellopontine angle, temporal bone, and skull base. | |
• Chest x-ray: sarcoidosis, lymphoma, carcinoma. |
Ultrasound assessment.
Mean facial nerve diameter was significantly larger in patients than in controls, with a significant side-to-side difference in patients. ROC curve analysis of the absolute facial nerve diameter showed a sensitivity of 75% and a specificity of 70% (59).
Special laboratory tests.
• Complete blood count: detect infectious mononucleosis or leukemia. | |
• Fasting blood glucose and 2-hour postprandial blood glucose for diabetes mellitus. | |
• Mono spot test or Epstein-Barr virus titers to detect infectious mononucleosis. | |
• Erythrocyte sedimentation rate for sarcoidosis and collagen vascular disease. | |
• Antinuclear antibody test, extractable nuclear antigen test, rheumatoid factor test, and neutrophil cytoplasmic antibody test for collagen vascular disease based on suspected clinical type. | |
• Venereal disease research laboratory test and fluorescent treponemal antibody titer. | |
• Lyme serology or CSF IgM capture ELISA. | |
• HIV titer. | |
• Serum angiotensin-converting enzyme level to detect sarcoidosis. | |
• CSF cell count and protein for acute inflammatory demyelinating polyradiculoneuropathy, multiple sclerosis, meningitis, meningeal carcinomatosis, and lymphoma. |
Eye care. This is the primary concern in Bell palsy. Lubricating eye drops during the day and an ointment-like petrolatum at night are usually effective. Taping the eye closed when sleeping or using an eye patch that will not abrade the cornea may be required. Rarely, a temporary tarsorrhaphy may be used to protect the globe.
Prednisone, antivirals, and combination treatment. Studies indicate that prednisone therapy is of value in the treatment of Bell palsy, whereas antivirals have a more limited role (11) and are not recommended for subjects with mild or moderate Bell palsy. Antivirals may be more effective in subjects with Bell palsy who do not have hypertension or diabetes (29) but need to be given in combination with corticosteroids. There was no clear benefit from antivirals alone over placebo. A discussion of the literature supporting this recommendation is given below (19). The exact dose of prednisone to be used is uncertain. However, most studies have used approximately 50 mg/day for 10 days. Therapy should be started within 72 hours of diagnosis.
In the BELLS study (58), the purpose was to determine whether oral prednisolone or acyclovir, used separately or in combination, early in the course of Bell palsy improves the chance of recovery at 3 and 9 months. The study was a 2 x 2 factorial randomized double-blind trial. Patients were randomly assigned to treatment. Patients with moderate to severe facial paralysis (n = 496) were randomized to receive active preparations or placebo for 10 days: (1) prednisolone (50 mg per day) and acyclovir (2000 mg per day); (2) prednisolone and placebo; (3) acyclovir and placebo; and (4) placebo and placebo. The primary outcome was recovery of facial function assessed by the House-Brackmann scale. One half of patients initiated treatment within 24 hours of onset of symptoms, and the rest within 72 hours. Of the completed patients, 357 recovered by 3 months and 80 by 9 months, leaving 59 with a residual deficit. There were significant differences in complete recovery at 3 months between the prednisolone comparison groups (83.0% for prednisolone, 63.6% for no prednisolone). The number needed to treat (NNT) in order to achieve one additional complete recovery was 6 (95% CI: 4 to 9). There was no significant difference between the acyclovir comparison groups (71.2% for acyclovir and 75.7% for no acyclovir). Nine-month assessments of patients recovered were 94.4% for prednisolone compared with 81.6% for no prednisolone; the NNT was 8 (95% CI: 6 to 14). Further, in a prospective, randomized, double-blind, placebo-controlled trial, patients were treated with prednisolone within 72 hours of onset of Bell palsy to evaluate whether treatment start and age are related to outcome (03). A total of 829 patients were followed for 12 months. Patients treated with prednisolone within 24 hours and within 25 to 48 hours had significantly higher complete recovery rates than patients given no prednisolone. For patients treated within 49 to 72 hours of palsy onset, there were no significant differences. Recovery rates were higher in patients older than 40 years of age.
Similar results were reported in an earlier publication (57). Thus, in patients with Bell palsy, early treatment with prednisolone significantly improved the chances of complete recovery, but there was no evidence of a benefit of acyclovir given alone or an additional benefit of acyclovir in combination with prednisolone.
In another study, 150 patients with Bell palsy were randomly assigned to a prednisolone group or a prednisolone-valacyclovir group, in whom virologic examinations for herpes simplex virus-1 and varicella-zoster virus were performed. Reactivation of herpes simplex virus-1 or varicella-zoster virus was observed in 34% of the patients with Bell palsy. The effect of combination therapy with prednisolone and valacyclovir on recovery was better but not significantly higher than that with prednisolone alone (31). In a further study, the aim was to compare the short-term and long-term effects of prednisolone and valacyclovir in the recovery of the affected facial nerve in a large number of patients (17). In this randomized, double-blind, placebo-controlled, multicentre trial, patients aged 18 to 75 years treated within 72 hours of onset of Bell palsy were randomly assigned to receive placebo plus placebo; 60 mg prednisolone per day for 5 days then reduced by 10 mg per day (for a total treatment time of 10 days) plus placebo; 1000 mg valacyclovir three times per day for 7 days plus placebo; or prednisolone (10 days) plus valacyclovir (7 days). Follow-up was for 12 months. The primary outcome event was time to complete recovery of facial function, as assessed with a regional Sunnybrook scale score of 100 points. In 829 studied patients, time to recovery was significantly shorter in patients who received prednisolone compared with the patients who did not (hazard ratio 1.40, p< 0.0001). There was no difference in time to recovery between patients treated with valacyclovir and those not receiving the drug. Adverse events were similar in all treatment arms. The conclusion was that prednisolone shortened the time to complete recovery in patients with Bell palsy, whereas valacyclovir did not affect facial recovery. In general, randomized, double-blind, placebo-controlled studies have shown that adult patients with Bell palsy benefited from early treatment with prednisone. Childhood Bell palsy shows a good prognosis with or without corticosteroid treatment and specifically, there is no difference in prognosis between treated and untreated groups (63).
In comparison to these studies, in a large randomized study, prednisolone combined with valacyclovir was superior to either drug alone when given within 7 days of onset of Bell palsy (21). In this study, 221 patients were treated within 7 days of the onset of Bell palsy and followed up until complete recovery occurred, or for more than 6 months in cases with a poor prognosis. The overall rate of patient recovery among all patients treated with valacyclovir and prednisolone (96.5%) was significantly better (p < 0.05) than the rate among those treated with prednisolone alone (89.7%). In cases of complete or severe palsy, the rates of patients treated with valacyclovir/prednisolone and prednisolone alone who recovered were 95.7% (n = 92) and 86.6% (n = 82), respectively; the recovery rate for treatment with valacyclovir and prednisolone was significantly better than that with prednisolone alone (p < 0.05). Eligible patients were randomly assigned to receive either valacyclovir 1000 mg per day for 5 days or placebo administered orally twice daily. All patients were simultaneously treated with oral prednisolone in gradually decreasing dosages: 60 mg per day for 5 days, 30 mg per day for 3 days, and 10 mg per day for 2 days. Furthermore, with more severe Bell palsy, the chance of reaching normal function was greater if patients were treated with famciclovir additionally, instead of with prednisone alone (73.7% vs. 47.1%; Cochran-Armitage trend test, p = 0.03). Combined treatment with famciclovir and prednisolone should be considered in patients with severe Bell palsy (38).
A meta-analysis combined direct and indirect comparisons for assessing efficacy of steroids and antiviral treatment (AVT) at 3 and 6 months (40). There were 1805 subjects, and the pooled odds ratios for resolution at 3 months were 1.24 (95% CI: 0.79 to 1.94) for acyclovir plus prednisolone and 1.02 (95% CI: 0.73 to 1.42) for valacyclovir plus prednisolone, versus prednisolone alone. Either acyclovir or valacyclovir singly had significantly lower efficacy than prednisolone alone, ie, odds ratios were 0.44 (95% CI: 0.28 to 0.68) and 0.60 (95% CI: 0.42 to 0.87), respectively. Neither of the antiviral agents was significantly different compared with placebo. Overall, prednisolone-based treatment increased the chance of recovery 2-fold (95% CI: 1.55 to 2.42).
Prednisone also reduces long-term sequelae of Bell palsy. In a prospective, randomized, double-blind, placebo-controlled multicenter trial with 12 months of follow-up, with 829 patients aged 18 to 75 years, prednisolone significantly reduced mild and moderate sequelae in Bell palsy (06).
In a head-to-head open-label, randomized trial, patients with acute Bell palsy were randomized into two groups. One group received a single dose (500 mg) of intravenous methylprednisolone, whereas the other group received 10 days of oral prednisone. In this study, intravenous methylprednisolone and oral prednisolone showed equivalent benefit in patients with acute Bell palsy (20).
In a double-blind, placebo-controlled, randomized trial, children aged 6 months to 18 years with Bell palsy were recruited within 72 hours after symptom onset (04). They were randomly assigned to receive 10 days of treatment with oral prednisolone (approximately 1 mg/kg) or placebo. The primary outcome was complete recovery of facial function at 1 month rated on the House-Brackmann scale. Secondary outcomes included facial function, adverse events, and pain for up to 6 months. A total of 187 children were randomized (94 to prednisolone and 93 to placebo) and included in the intention-to-treat analysis. Most children with Bell palsy recovered without treatment. The study was underpowered and did not show that early treatment with prednisolone improved complete recovery.
A meta-analysis of eight randomized clinical trials conducted between 1994 and 2011 with 1315 patients compared use of oral corticosteroids alone with the addition of acyclovir, valacyclovir, or famciclovir to oral corticosteroids for the treatment of Bell palsy. Addition of the antiviral therapy to the corticosteroids was associated with a higher proportion of people who recovered at the 3- to 12-month follow-up. However, using the antiviral alone was not beneficial. The quality of evidence, however, was limited by heterogeneity, imprecision of the result estimates, and risk of bias (56).
Thus, in conclusion, the studies indicate that prednisone is beneficial in reducing the severity of Bell palsy and increasing the rate of recovery. A reasonable protocol is to use 1 mg/kg prednisone per day (up to 50 mg) for 10 days. To be of any value, it should be started within 72 hours of clinical onset, ie, before neuronal denervation is established. Adding an antiviral to prednisone provides some benefit in terms of outcome. It is not known if a specific antiviral shows superiority over others.
A further question concerns the effect of prednisone and antivirals on long outcomes when used in patients presenting within 72 hours of the onset of Bell palsy. Compared with oral corticosteroids alone, the addition of acyclovir, valacyclovir, or famciclovir to oral corticosteroids for treatment of Bell palsy was associated with a higher proportion of people who recovered at a 3- to 12-month follow-up (56).
Eyelid surgery. This is reserved for patients with severe palsy or where there is anesthesia of the cornea or xerophthalmia not amenable to eye lubricants. Suture tarsorrhaphy is effective but limits vision, may lead to ingrowth of eyelashes resulting in further irritation of the cornea, and can create a cosmetic defect. Gold-weighting of the upper eyelid provides an alternative therapy and can be removed as the facial nerve regenerates.
Surgical decompression of the facial nerve. The role of surgical decompression as a therapy for Bell palsy is controversial. The main role for surgical exploration is in patients where the diagnosis of Bell palsy is in doubt, ie, when: (1) a history of trauma exists or the onset is sudden and complete with an absent facial evoked response; (2) where clinical evaluation and radiological studies suggest the presence of a malignant infiltration of the nerve; (3) when the facial paralysis progresses over more than 1 month; (4) when no recovery whatsoever is seen after 1 year; and (5) when progression of other cranial nerve deficits occurs.
Surgical options include selective myectomy, selective neurectomy, cross-facial nerve grafting, nerve substitution, and free gracilis muscle transfer. The Canadian Neurological Sciences Federation is against the routine use of surgical decompression. They further recommend that patients should consider this option only if they have severe facial nerve degeneration on electroneuronography, if they are willing to accept the surgical risks, and if the surgery is to be performed in a tertiary referral center with special expertise in this procedure (11).
Botulinum toxin injections. Botulinum toxin may be useful in symptomatic synkinesis or spasm (18) or for hyperlacrimation secondary to an aberrant regenerated seventh nerve palsy (32). Oculo-oral synkinesis may be more responsive than oro-ocular synkinesis (54).
Facial reanimation. Synkinesis is commonly encountered after flaccid facial paralysis and can adversely affect patients. First-line treatment of synkinesis is chemo-denervation with botulinum toxin and neuromuscular retraining. In a few cases of Bell palsy, recovery does not occur by 1 year, and in some of these cases, exploration of the facial nerve has shown evidence of atrophy and fibrosis of the nerve. Thus, in some patients who have otherwise typical features of Bell palsy but little or no improvement after 1 year, surgical exploration of the nerve and reanimation of the face may be indicated. Reanimation techniques include end-to-end anastomosis of the facial nerve; sural, hypoglossal, or greater auricular nerve grafts; and a variety of nerve pedicle transpositions from the cervical, temporalis, or masseter region. Selective neurectomy may be useful in some for treatment of post-facial paralysis synkinesis(53).
Acupuncture. An analysis of acupuncture trials concluded that poor quality study design and the low numbers of trials make it difficult to determine if acupuncture is effective (07; 34).
The incidence of Bell palsy in pregnancy and the puerperium ranges from 38 to 45 per 100,000 live births, compared to 17 per 100,000 live births in nonpregnant women of childbearing age. Older literature suggests that pregnant women are 3.3 times more likely to develop Bell palsy than nonpregnant women, particularly in the third trimester (23); however, these results may have been prone to attainment bias. However, newer data from a national cohort study suggested that the annual incidence of Bell palsy was not increased in pregnant women relative to that in the control. The incidence of Bell palsy in both groups was measured from pregnancy to 1 year postpartum. Among the 63,264 pregnant/postpartum participants, 20 were diagnosed with or treated for Bell palsy during pregnancy and 38 during postpartum periods (08). The annual incidence of Bell palsy per 100,000 women during pregnancy was 43.4 in the patient group and 80.2 in the control group (p < 0.05) and during postpartum periods was 60.1 in the patient group and 50.6 in the control group (p > 0.05).
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

James W Russell MD MS
Dr. Russell of the University of Maryland has no relevant financial relationships to disclose.
See Profile
Louis H Weimer MD
Dr. Weimer of Columbia University has received consulting fees from Roche.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Peripheral Neuropathies
Jul. 17, 2024
Neuro-Ophthalmology & Neuro-Otology
Jun. 18, 2024
Neuro-Oncology
May. 28, 2024
Peripheral Neuropathies
May. 28, 2024
Neuroimmunology
May. 24, 2024
Neuropharmacology & Neurotherapeutics
May. 20, 2024
Neuro-Ophthalmology & Neuro-Otology
May. 20, 2024
General Neurology
May. 15, 2024