Neurobehavioral & Cognitive Disorders
Transient global amnesia
Jul. 19, 2024
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Most of the signs and symptoms of carbon monoxide poisoning are due to hypoxia. The most significant neurologic and psychiatric manifestations of carbon monoxide poisoning are subacute or late sequelae, often following a period of apparent recovery from an acute episode. Carbon monoxide exposure causes oxidative stress that triggers activation of N-methyl-D-aspartate--neuronal nitric oxide synthase pathway, a key factor in the progression of carbon monoxide–mediated neuropathology. The most important diagnostic test for carbon monoxide poisoning is the direct spectroscopic measurement of carboxyhemoglobin level in the blood. Hyperbaric oxygen is an important component of the management of carbon monoxide poisoning.
|
• Most of the effects of carbon monoxide poisoning are due to hypoxia. | |
|
• Neurologic sequelae of carbon monoxide poisoning may be delayed in onset. | |
|
• Hyperbaric oxygen plays an important role in managing carbon monoxide poisoning. |
Humans have been exposed to carbon monoxide since they first made fire inside sheltered caves. In 300 BC, Aristotle's comment that "coal fumes lead to a heavy head and death" was a reference to carbon monoxide poisoning. In the 19th and 20th centuries, the increased burning of coal greatly increased the frequency of carbon monoxide poisoning.
Early recognition of the features of carbon monoxide poisoning. The bright red skin complexion in victims who died from charcoal fumes (much later recognized as resulting from elevated carboxyhemoglobin levels) was documented by Swiss pathologist and pharmacologist Johann Jakob Wepfer (1620-1695) in the 1600s and by French anatomist, physician, and medical historian Antoine Portal (1742-1832) in the late 1700s.
Line engraving by J P Dupin Jr, 1782, after André Pujos (1738-1788), 1781. (Courtesy of the Wellcome Collection, London, England. Creative Commons Attribution 4.0 International License, https://creativecommons.org/licenses/by/4...
The first clinical description of coal gas poisoning (ie, carbon monoxide poisoning) was by French physician Dominique-Benoît Harmant (1723-1782) from Nancy, France, in 1775 (50).
Early chemistry of carbon monoxide. Around 1772, English chemist Joseph Priestley (1733-1804) isolated carbon monoxide, which he called "heavy inflammable air" (14).
Thomas Beddoes and James Watt recognized "hydrocarbonate" ("water gas" generated by passing steam over coke, which generates carbon monoxide and hydrogen) brightened venous blood in 1793. Watt suggested coal fumes could counteract the oxygen in the blood, and in 1796 Beddoes and Watt speculated hydrocarbonate has a greater affinity for animal fiber than oxygen.
In 1800, Scottish military surgeon and chemist William Cruickshank (born circa 1740 or 1750, died 1810 or 1811) identified carbon monoxide as a compound containing carbon and oxygen.
Early concepts of the pathophysiology of carbon monoxide poisoning. German physician and physiologist Johann Joseph Dömling (1771-1803) in 1803 and English physician and geologist John Bostock (1773-1846) in 1804 suggested that blood returned to the heart loaded with carbon monoxide to subsequently be oxidized to carbon dioxide in the lung prior to exhalation.
A half-century later, in 1854, Adrien Chenot (1803-1855) similarly suggested that carbon monoxide could remove oxygen from the blood and be oxidized within the body to carbon dioxide; sadly, Chenot died suddenly and unexpectedly in November 1855 after falling out of a window, apparently due to disorientation and nausea from experimenting with carbon monoxide poisoning.
In 1865, German-Swiss microbiologist Theodor Albrecht Edwin Klebs (1834-1913) described clinical and pathologic findings in rats exposed to carbon monoxide (62).
The interaction of carbon monoxide and hemoglobin. In 1857, French physiologist Claude Bernard (1813-1878) showed that carbon monoxide produces hypoxia by reversible combination with hemoglobin (11). In his memoirs, beginning in 1846 and published in 1857, Bernard notably concluded that carbon monoxide "prevents arterial blood from becoming venous."
Lithograph by Alexandre Laemlein (1813-1871). (Source: Bibliothèque interuniversitaire de Santé, Paris. Licence Ouverte 1.0, https://en.wikisource.org/wiki/Open_Licence_v1.0. Illustration restored by Dr. Douglas J Lanska.)
In 1858, German physiologist and chemist Felix Hoppe-Seyler (1825-1895) independently published similar conclusions to Bernard’s and also developed the first analytical method to detect carboxyhemoglobin. The first quantitative analysis method for carbon monoxide poisoning was developed by German public health physician Josef von Fodor (1843-1901) in 1880.
The strong affinity of carbon monoxide for hemoglobin, first suspected by Bernard, was confirmed by Scottish physician and physiologist John Scott Haldane (1860-1936) in 1895 (43; 44).
Haldane showed that rats survived carbon monoxide poisoning when placed in oxygen at a pressure of two ATA (ATA means a pressure reading in technical atmosphere units ["at"], which are referenced to a perfect vacuum [absolute]). Haldane suggested that introducing oxygen to organisms subjected to carbon monoxide poisoning might improve the condition (43; 44), which he elaborated on in subsequent publications (44; 45; 47; 46).
In 1896, in an extremely influential report, Haldane presented his assessment of the causes of death in colliery explosions (ie, explosions in coal mines) (46). In what was then a very novel finding, Haldane concluded that most of the deaths in several devastating British mine explosions were attributable to carbon monoxide poisoning and not asphyxia from inadequate oxygen or traumatic effects of the explosions (47). In the only color plate in his official report, Haldane contrasted the color of a solution of normal blood, normal blood shaken with coal gas (containing a mixture of hydrogen, methane, carbon monoxide, and ethylene), and the blood of one of the fatalities; the progressively saturated red color was due to increasing content of carboxyhemoglobin.
A solution of normal blood (top), normal blood shaken with coal gas (containing a mixture of hydrogen, methane, carbon monoxide, and ethylene) (middle), and the blood of one of the fatalities of a coal mine explosion in Britain...
Subsequently, in 1912, the binding of hemoglobin and carbon monoxide was described in more detail by British physiologist Claude Gordon Douglas (1882-1963), JS Haldane, and Haldane's son, John Burdon Sanderson Haldane (nicknamed "Jack" or "JBS") (28). They constructed hemoglobin-carbon monoxide dissociation curves, illustrating how the amount of inspired oxygen is related to the saturation of hemoglobin with carbon monoxide.
Although the curve for each individual is a rectangular hyperbola, the percentage saturation with a given percentage of carbon monoxide varies for different individuals and across species. However, the dissociation curves are n...
The points determined lie on the rectangular hyperbolas drawn through them until the dotted portions of the curves are reached for mice with very low percentages of oxygen and carbon monoxide. "The existence of the hump points ...
The different curves are almost identical if the scale on which the abscissae of each are plotted is altered by a suitable constant. Thus, the effect of varying proportions of CO2, (and presumably of acidity or alkalinity in ge...
Haldane and colleagues found that when a solution of hemoglobin (enclosed in red blood corpuscles or free) is saturated in the presence of a gas mixture containing oxygen and carbon monoxide, the ratio of oxyhemoglobin to carboxy-hemoglobin is proportional to the relative partial pressures of oxygen and carbon monoxide but varies across individuals and species. The ratio is not altered by the presence of CO2 or of reduced hemoglobin, nor by dilution, but is appreciably altered by temperature.
In 1927, JBS Haldane further determined that carbon monoxide not only disrupts hemoglobin's oxygen-carrying capabilities but also poisons specific tissues (28).
Central nervous system complications of carbon monoxide poisoning. In the 1920s, particularly in Germany, Hungary, and the United States, carbon monoxide was linked to preferential damage to the internal segment of the globi pallidi, with a range of accompanying neuropsychiatric and extrapyramidal motor manifestations (101; 96; 93; 38).
The classic bilateral lesions of the globus pallidus and diffuse subcortical demyelination were described and linked with "psychic akinesia" by the German Jewish neurologist Hermann O Pineas (1892-1969) in Berlin in 1924 (93).
"Completely fatty small artery in a focus of softening. Details of the brain substance cannot be recognized anymore." Death occurred 10 days after poisoning. Staining with Sudan and hematoxilin (Sudan dyes, which are used to st...
(Source: Pineas H. Klinischer und anatomischer befund eines falles von co-vergiftung. Ein beitrag zur frage der psychomotorischen apraxie und verwandter bewegungsstörungen. Zeitschrift für die gesamte Neurologie und Psychiatrie...
Pineas survived the Holocaust by hiding from Nazi persecutors under an assumed name with forged documents and twice escaping from the Gestapo in 1943. In 1946, after World War II ended, he immigrated to New York and became a clinical neurologist at a Veteran's Administration clinic from 1952 to 1969. Pineas's "psychic akinesia" resembles what was labeled subcortical dementia in the late 20th century and has since been labeled by various other terms, including "auto‐activation deficit," "hypoactive-hypoalert behavior," and "athymhormia" (69; 70; 71; 109; 68; 42; 27; 106; 72; 73; 41; 118). Psychic akinesia is due to bilateral lesions in the brainstem, basal ganglia, or the subcortical frontal lobes and is characterized by extreme passivity, apathy, blunted affect, and a profound generalized loss of self-motivation that are reversed by external stimulation.
Carbon monoxide poisoning was linked with parkinsonism by American neuropsychiatrist Roy R Grinker (1900-1993), then an attending neurologist at Cook County Hospital in Chicago (38).
Abbreviations: cc., corpus callosum; Comm. ant., anterior commissure; n, necrotic area of pallidum. (From: Grinker R. Parkinsonism following carbon monoxide poisoning. J Nerv Ment Dis 1925;18:16.)
Grinker received his MD degree from Rush Medical College in 1921 and then spent a year in Europe before returning to the United States. In 1933, he returned to Europe for psychoanalytic training with Sigmund Freud. Most of his later career focused on psychiatry.
Selected 20th-century events. On his second Antarctic expedition in 1934, American naval officer and explorer Richard E Byrd (1888-1957) spent 5 winter months alone operating a meteorological station, "Advance Base," from which he narrowly escaped with his life after suffering carbon monoxide poisoning from a poorly ventilated stove. This expedition is described in Byrd's autobiography, Alone (1938).
The effectiveness of hyperbaric oxygen therapy in experimental carbon monoxide poisoning in dogs and guinea pigs was demonstrated in 1942 (31). In 1960, hyperbaric oxygen therapy was first used successfully in treating human cases (107).
As a counterpoint to the improved ability to resuscitate and manage victims of carbon monoxide poisoning, carbon monoxide was also used as a method of killing. The Nazis first began using poison gas for mass murder in December 1939, when an SS Sonderkommando unit used carbon monoxide for this purpose. In the 1990s, medical suicide advocate and pathologist Jack Kevorkian (1928-2011) used carbon monoxide as a euthanasia agent.
National Press Club, Washington, DC on July 29, 1996. (Source: Kingkongphoto and www.celebrity-photos.com, https://www.flickr.com/people/362770 35@N06, from Laurel, Maryland. Creative Commons Attribution-Share Alike 2.0 Generic...
A physiological role for carbon monoxide? Carbon monoxide is produced in small amounts endogenously during the catabolism of heme, resulting in the coproduction of biliverdin and iron. Carbon monoxide is a signaling molecule that shares some chemical and biological properties with nitric oxide and is a mediator in the autonomic nervous system. Actions of carbon monoxide in the nervous system, thus, range from the physiological to the pathological.
|
• Most of the signs and symptoms of carbon monoxide poisoning are due to hypoxia, which affects mainly the brain, but other vital organs, such as the heart, are also involved. | |
|
• Severe poisoning (carboxyhemoglobin 40% to 60%) may lead to coma, convulsions, and respiratory impairment. | |
|
• Late sequelae of carbon monoxide poisoning include dementia, parkinsonism, and psychoses. |
The onset of carbon monoxide poisoning may be acute, or it may be insidious with chronic, low-grade poisoning. Most of the signs and symptoms of acute carbon monoxide poisoning are due to hypoxia, which affects mainly the brain, but other vital organs (eg, the heart) may also be involved (Table 1). The severity of acute symptoms usually corresponds to the blood carboxyhemoglobin level, but frequent disparities exist.
|
• Dull headache |
Mild carbon monoxide poisoning. The most frequent neurologic manifestations of mild acute carbon monoxide poisoning (carboxyhemoglobin 10% to 20%) are headache (90%), dizziness (82%), and visual disturbances. These may be accompanied by impairment of higher cerebral function, weakness, nausea, and abdominal pain.
Moderate carbon monoxide poisoning. With moderate carbon monoxide poisoning (carboxyhemoglobin 20% to 40%), the patient may present with cardiac disturbances, dyspnea, and vomiting. Loss of consciousness may occur.
Severe carbon monoxide poisoning. Severe carbon monoxide poisoning (carboxyhemoglobin 40% to 60%) often presents with coma, convulsions, and respiratory impairment. A vegetative state, extrapyramidal rigidity, movement disorder, tic disorder, or severe memory deficits may follow recovery from coma.
Attempts to relate specific symptoms to specific carboxyhemoglobin levels are problematic because none of the symptoms of carbon monoxide poisoning can be associated with a specific carboxyhemoglobin level (49).
Cardiac toxicity can present with chest pain, myocardial ischemia, ventricular arrhythmia, acute congestive heart failure, and severe metabolic lactic acidosis. Thus, cerebral symptoms may occur in combination with a non-Q/non-ST elevation myocardial infarction, with associated enzyme elevations and electrocardiographic abnormalities. Other manifestations in comatose patients include neurogenic pulmonary edema and acute renal failure due to muscle necrosis or carbon monoxide-induced rhabdomyolysis without pressure necrosis.
Children seem to have a lower threshold for toxicity of carbon monoxide. In children, lethargy and syncope may occur at a mean carboxyhemoglobin concentration of 25%, whereas these symptoms are seen in adults at a level of about 40% carboxyhemoglobin.
Chronic carbon monoxide exposure. Symptoms of chronic exposure to carbon monoxide are typically vague and nonspecific but may include headache, fatigue, dizziness, paresthesias, chest pain, palpitations, and visual disturbances. Some chronic poisoning cases may produce mild manifestations that do not precipitate medical evaluation or recognition of the underlying problem ("occult carbon monoxide poisoning"). "Warehouse worker headache" is a term coined for carbon monoxide poisoning from industrial exposure to exhausts in unventilated indoor environments.
Some patients presenting with the complaint of headache to an emergency department, particularly during winter-heating months, have raised carboxyhemoglobin levels due to carbon monoxide exposure.
Delayed neurologic sequelae. Subacute or late neurologic sequelae often follow a period of seemingly complete recovery from acute poisoning (Table 2). These are also referred to as the "interval form of carbon monoxide poisoning," "secondary syndromes," or "delayed postanoxic encephalopathy." Such delayed manifestations typically develop 1 to 3 weeks after exposure to carbon monoxide. In some cases, there has been a series of repeated exposures to carbon monoxide.
Among the survivors of acute carbon monoxide poisoning, the incidence of secondary syndromes varies from 15% to 40%.
Several movement disorders occur as late sequelae of carbon monoxide poisoning, mostly in association with neuropsychiatric deficits (Table 1). Delayed movement disorders, such as parkinsonism, are the most prominent of these.
|
Disorders of arousal, attention, global cognitive function | |
|
• Vegetative state, apallic syndrome, or unresponsive wakefulness syndrome | |
|
Convulsive disorders | |
|
Cerebrocortical dysfunction (focal or restricted) | |
|
• Memory disturbances | |
|
Psychiatric conditions | |
|
• Apathy | |
|
Headaches | |
|
Movement disorders | |
|
• Akinesia or hypokinesia | |
|
Optic neuritis | |
|
Deafness (neural) | |
|
Urinary incontinence | |
|
Peripheral neuropathy | |
Children are subject to the same neuropsychiatric sequelae that are seen in adults. Developmental plasticity of the brain may explain a better prognosis for functional recovery in children with carbon monoxide-induced neuropsychological impairment.
Clinical diagnosis of carbon monoxide poisoning is based more on the history of exposure than on the clinical examination findings. The classic cherry-red color of the skin is rarely seen in nonfatal cases. Carbon monoxide is a colorless, odorless, and tasteless gas; the only evidence of exposure to it may be the elevated carboxyhemoglobin levels in the blood, but the severity of signs and symptoms does not always correspond to the measured carboxyhemoglobin level. Indeed, in cases with delayed manifestations, carboxyhemoglobin levels may no longer be elevated.
Carboxyhemoglobin levels of less than 10% are usually considered clinically insignificant, but several studies on human volunteers have shown subtle changes on neuropsychological testing. Some of these changes are decreased cognitive and psychomotor ability with carboxyhemoglobin levels of 2% to 5% and impaired ability to discriminate short time intervals with carboxyhemoglobin levels of 4% to 5%.
The mortality of acute carbon monoxide poisoning is about 30% in untreated cases. It is reduced considerably in patients treated by hyperbaric oxygen therapy. Mortality is high in patients who remain in a coma beyond 48 hours, and the survivors have severe neurologic deficits. The prognosis for recovery is usually poor in patients who remain in a "vegetative state" for more than 1 month following carbon monoxide poisoning. Still, good functional recovery has been reported in exceptional cases after remaining in such a state for a few months. Level of carboxyhemoglobin is not a reliable prognostic index, but blood pH may be a significant factor.
There appear to be no biomarkers or constellation of signs or symptoms at presentation that predict long-term outcome following carbon monoxide poisoning. Interleukin-6 in CSF at the early phase of carbon monoxide poisoning may be a predictive biomarker of delayed encephalopathy (54). The prognosis is usually good in most cases with a normal CT, and the chances for recovery diminish in patients with abnormal CT, especially when lesions of both the globus pallidus and the white matter are evident.
Patients with severe carbon monoxide poisoning who have abnormalities on CT that persist after hyperbaric oxygen treatment are more likely to develop neuropsychiatric sequelae. More than half of patients are diagnosed as suffering from cognitive impairments and other neurologic symptoms following carbon monoxide poisoning, with affective disorders in almost three fourths of these patients and personality disorders in more than half (13).
The risk for developing adrenal insufficiency is elevated after carbon monoxide poisoning, especially in females and in younger patients (52). In a retrospective cohort study using the Taiwan Nationwide Poisoning Database, the 21,842 patients with carbon monoxide poisoning diagnosed between 1999 and 2012 had a higher risk for adrenal insufficiency than the 43,684 reference participants (adjusted hazard ratio: 2.5; 95% CI: 1.8–3.5) after adjustment for sex and underlying comorbidities (liver disease, thyroid disease, mental disorder) (52); the risk continued to increase even after 1 year.
Hungarian neurologist and neurohistologist Hugó Richter (1887-1945), working under anatomist and neurologist Károly Schaffer (1864-1939) in Budapest, included two cases of carbon monoxide poisoning in a series of extrapyramidal movement disorders (96). Although these are historic cases, they provide excellent examples of the clinical features of carbon monoxide poisoning with corresponding neuropathology.
Case 1. The following case is from a 62-year-old widow, Mrs. JF, who was admitted to the hospital in November 1919 after collapsing and losing consciousness in front of a gas oven 8 days earlier. She remained unconscious from 6 pm until the next morning when relatives found her with multiple burns on her foot. There was a strong smell of coal gas in the room, produced by the coal gasification reaction, with the burnable component consisting of a roughly equal molecular mixture of carbon monoxide and hydrogen. Afterward, she was extremely weak, had difficulty thinking, was always sleepy, and could only walk with support.
Cognitive function was severely impaired. Questions had to be repeated four to five times until she could understand them; she then usually repeated the question and gave a mostly correct answer in a few words. She had no memory of her illness and admission, and her memory of earlier experiences was also noticeably impaired. She could recognize individual objects placed in front of her but often named them with a description of their use (eg, when shown a key she replied "open"). She could not perform simple multiplication: when asked to state the product of 5 x 5, she replied, "five hundred and twenty-five"; for the product of 5 x 20, she replied, "five hundred." She also manifested palilalia.
She had masked facies, rarely blinked, was diffusely rigid, and had a markedly stooped posture when standing with her arms fixed in an "obstetrician position" (ie, the position of the arms as if delivering a baby). In addition, she had a small-stepped, festinating gait, and needed support to walk.
She lay motionless in bed for hours. If her arms were passively moved, she maintained these unusual positions for extended periods, resembling the cerea flexibilitas of the catatonic. She displayed no protection or defense movements to stimuli. If she began an active movement with her arm when repeatedly asked to do so, she often stopped during the execution and only continued in the desired direction when persuaded to do it again; sometimes, this was not completed despite being asked to do so, and the arm remained fixed in a pointless position for several minutes.
Tendon reflexes were brisk (spastic), particularly on the right side, but Babinski, Mendel (ie, dorsal flexion of the second to fifth toes on percussion of the dorsum of the foot), Oppenheim, and Gordon's signs were absent. The great toes of both feet were sometimes spontaneously maintained in a position of dorsiflexion (ie, the "pseudo-Babinski sign" of Oskar and Cécile Vogt). Abdominal reflexes could not be elicited. There were no sensory disturbances.
Over the following 2 months, she suffered bronchopneumonia, progressive marasmus, and sacral decubiti with osteomyelitis before ultimately dying in January 1920.
Case 2. A 56-year-old widow, Mrs. CH, was admitted in June 1920 with a disturbed sensorium. According to her relatives, she fell ill 4 days previously from coal gas poisoning; although she had not completely lost consciousness, she had impaired cognition and recurrent vomiting afterward.
She had masked facies, rarely blinked, and was diffusely rigid. Her speech was monotonous and sometimes indistinct. She had intermittent difficulty swallowing. Her arms were held in a parkinsonian position: adducted at the shoulder, slightly bent at the elbow, and pronated. Positions once taken were retained for a long time. Active movements were rare, very slow, and proceeded with interruptions. She could neither stand nor walk. Babinski and Oppenheim signs were consistently present on the right but inconsistent on the left. Clonus was absent. There were no sensory disturbances.
The patient was of short stature and well-nourished. Her bone system and joints were normal. There was gland swelling and pale skin and mucous membranes. A dampened percussion sound was observed over the right lung from the tip of the shoulder blade downwards; also observed were crepitation, bronchial breathing, and increased bronchophonia. The left lung gave way when breathing, with dry noises. Cardiac arrest was normal. Pulse of small waves was easily suppressed, p. M. 100. Her temperature was 38.60 C. Her tongue was dry and coated. Her abdomen was not painful and showed no resistance. The borders of the liver and spleen were normal. In the urine, there was no albumen and no sugar. Blood counts were as follows: 3,600,000 red blood cells; 3,300 white blood cells; Hgbl: 66. Qualitative measures were: 76% leukocytes, 8% small, 4% large lymphocytes, 6% transitional cells, 4% eosinophils, and 2% myelocytes.
Her level of arousal and attention changed rapidly, but at times her sensorium cleared, and she was able to give correct answers to simple questions. Sometimes she suddenly started crying without evident provocation. She seemed to have no memory of her illness. She sometimes took notice of her relatives, but she mostly remained apathetic, mute, and motionless in bed.
With progression of a right-sided pan-lobar pneumonia, she developed increasing drowsiness and rigidity, with episodic periods of mental restlessness with violent crying and screaming. Babinski and Oppenheim signs were present bilaterally. She died in July 1920, about a month after suffering from carbon monoxide poisoning.
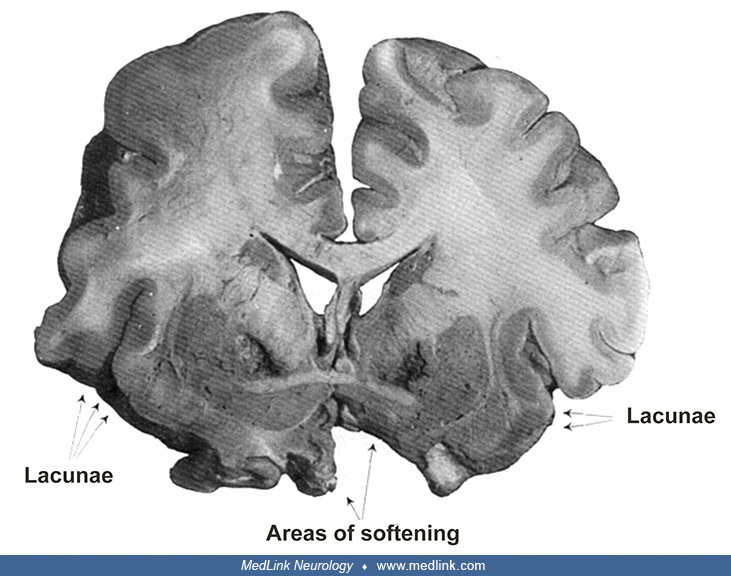
Pathology. In both cases, the neuroanatomical changes "showed such an extensive similarity both in the macroscopic and in the histological picture that a separate description of them is not indicated at all." There was a bilaterally symmetrical softening in the innermost part of the pallidum (ie, the internal segment) (96).
The external segment of the pallidum is intact, as are the putamen and caudate nucleus. The inner capsule limits the focus medially. (Source: Case 1 of: Richter H. Beiträge zur Klinik und pathologischen Anatomie der extrapyram...
Focus of softening or necrosis in the inner part of the pallidum. (Source: Case 2 of: Richter H. Beiträge zur Klinik und pathologischen Anatomie der extrapyramidalen Bewegungsstörungen [Contributions to the clinical and patho...
The foci of necrosis were intensely yellow in color, their walls irregularly hollowed out. These necrotic foci were sharply demarcated medially by the bundles of the inner capsule that appeared intact, inferiorly by the anterior commissure, and laterally by the outer pallidum (ie, external segment) that remained apparently healthy.
(Source: Case 1 [figure 10b] of: Richter H. Beiträge zur Klinik und pathologischen Anatomie der extrapyramidalen Bewegungsstörungen [Contributions to the clinical and pathological anatomy of extrapyramidal movement disorders....
(Source: Case 1 [figure 11b] of: Richter H. Beiträge zur Klinik und pathologischen Anatomie der extrapyramidalen Bewegungsstörungen [Contributions to the clinical and pathological anatomy of extrapyramidal movement disorders....
|
• Important causes of carbon monoxide poisoning are exposures to automobile exhaust, domestic cooking gas, industrial plant exhausts, and fire smoke. | |
|
• Inhaled carbon monoxide diffuses readily across the alveolar-capillary membrane and binds to hemoglobin to form carboxyhemoglobin. | |
|
• The affinity of carbon monoxide for hemoglobin is 200 to 300 times greater than the affinity of oxygen for hemoglobin. | |
|
• Deleterious effects of carbon monoxide levels are often related to hypoxia, but other processes are also involved. | |
|
• Carbon monoxide specifically inhibits cytochrome c oxidase of the human mitochondrial respiratory chain. | |
|
• The pathogenesis of acute carbon monoxide poisoning at a cellular and tissue level involves multiple processes, including apoptosis, abnormal inflammatory responses, hypoxia, and ischemia or reperfusion-like injury. | |
|
• Carbon monoxide poisoning may produce oxidative injury by reactive oxygen species (free radicals) and neuronal nitric oxide. |
Carbon monoxide is an odorless, tasteless, and nonirritating gas produced by the incomplete combustion of organic matter.
Carbon and oxygen together have a total of 10 electrons in the valence shell. When the two atoms form a triple bond, there are six shared electrons in three bonding molecular orbitals. Because four of the shared electrons come from the oxygen atom and only two from carbon, one bonding orbital is occupied by two electrons from oxygen (a dative or dipolar bond). This causes a C←O polarization, with a small negative charge on carbon and a small positive charge on oxygen.
In a poorly ventilated area, people exposed to carbon monoxide can quickly develop life-threatening toxicity. Because of shared exposures, cases are often clustered in families or groups of workers.
Traces of carbon monoxide occur in the atmosphere, and some carbon monoxide is produced endogenously, but these amounts are ordinarily insignificant. Important causes of carbon monoxide poisoning are exposure to automobile exhaust, domestic cooking gas, industrial plant exhausts, fire smoke, and cigarette smoke. Suicide attempts may involve inhalation of cooking gas or automobile exhaust.
Poisoning can occur because of faulty or improperly ventilated fuel-fired equipment (eg, stove, furnace, hot water heater, refrigerator, indoor pool or spa heater, wood burning fireplace, gas log burner, unvented space heater); indoor use of heaters, generators, or other internal combustion engines; gas or fuel-burning appliances in cabins or campers; and indoor use of charcoal grills.
Unintentional carbon monoxide poisoning is more common during winter and cold spells and when electric power is interrupted.
Exposure to methylene chloride (also known as dichloromethane) in poorly ventilated areas without protective respirators or gloves poses a risk of inadvertent carbon monoxide poisoning because of the metabolism of methylene chloride to carbon monoxide in the liver (26; 67; 65; 66; 09; 07; 111; 08; 33; 110; 112; 97; 98; 105; 100; 75; 82; 87; 81; 30; 63; 80; 12). Methylene chloride is found in many domestic and industrial products, including paint strippers, fixatives, varnishes, Christmas bubble lights, and spray paint. Metabolism of methylene chloride produces carbon monoxide via a cytochrome P450 mixed-function oxidase. Methylene chloride toxicity, like methanol toxicity, results from toxic metabolites rather than from direct intrinsic neurotoxicity of methylene chloride itself, a classic example of "lethal synthesis," "suicide metabolism," or a chemical "Trojan horse."
Most inadvertent carbon monoxide fatalities are from smoke inhalation. Carbon monoxide is the most common killer in fires and smoke inhalations. Automobile exhaust is the most significant source of atmospheric pollution with carbon monoxide. Smoking is the commonest and the most underestimated cause of nonfatal chronic carbon monoxide intoxication.
Factors contributing to carbon monoxide poisoning are high carbon monoxide concentration in the air being breathed, prolonged exposure, and high altitude with low oxygen tension.
Physical exercise and restlessness can increase carbon monoxide intake at the site of exposure. Carbon monoxide poisoning is aggravated by several preexisting medical conditions, such as anemia, myocardial ischemia, cerebrovascular disease, thyrotoxicosis, fever, and diabetes. Hyperglycemia following carbon monoxide poisoning is associated with poor outcomes. By increasing glucose availability, hyperglycemia presumably increases cerebral glycolytic activity and elevates brain intracellular lactate levels that induce acidosis and edema.
Pathogenesis of acute carbon monoxide poisoning. Acute carbon monoxide poisoning involves multiple cellular and tissue processes, including apoptosis, abnormal inflammatory responses, hypoxia, ischemia or reperfusion-like injury, and oxidative injury induced by reactive oxygen species, free radicals, and neuronal nitric oxide (01).
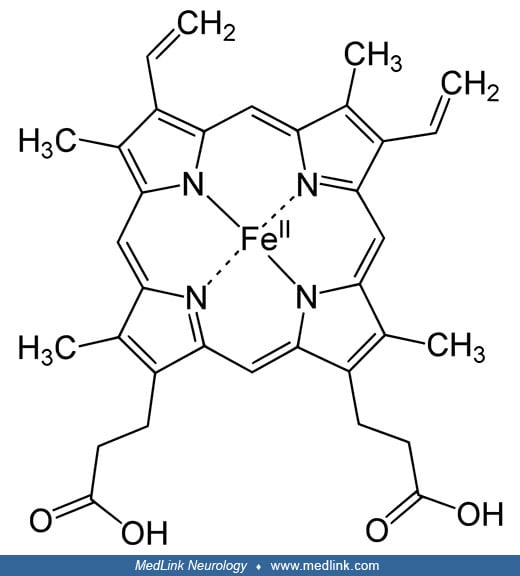
Inhaled carbon monoxide diffuses readily across the alveolar-capillary membrane and binds to the iron ions in the heme groups of hemoglobin to form carboxyhemoglobin.
(Source: Vasquez GB, Ji X, Fronticelli C, Gilliland GL. Human carboxyhemoglobin at 2.2 A resolution: structure and solvent comparisons of R-state, R2-state and T-state hemoglobins. Acta Crystallogr D Biol Crystallogr 1998;54(Pt...
The affinity of carbon monoxide for hemoglobin is 200 to 300 times greater than that of oxygen. Deleterious effects of elevated body carbon monoxide levels are often called carbon monoxide-hypoxia; the term implies that an inhibition of oxygen transport occurs from the blood to the tissues. Tissue oxygen tension may be decreased directly through a reduction in oxygen content by a lowered arterial oxygen tension as well as through the presence of carboxyhemoglobin. The oxygen dissociation curve is shifted to the left in the presence of carbon monoxide.
Although the clinical effects of carbon monoxide are usually attributed to tissue hypoxia alone, they do not always correlate with carboxyhemoglobin levels. The neuropathologic sequelae of carbon monoxide poisoning cannot be explained by hypoxia alone. Instead, carbon monoxide toxicity results from a combination of tissue hypoxia-ischemia secondary to carboxyhemoglobin formation and direct carbon monoxide-mediated damage at a cellular level (02; 40).
In addition to its binding to hemoglobin, carbon monoxide binds to myoglobin, cytochromes, and NADPH reductase, impairing oxidative phosphorylation in the mitochondria of the cells. Carbon monoxide specifically binds to and inhibits cytochrome c oxidase (complex IV) of the human mitochondrial respiratory chain. Consequently, carbon monoxide alters brain oxidative metabolism by mechanisms independent of the carboxyhemoglobin-related decrease in oxygen delivery (02). Extended and generalized inhibition of mitochondrial cytochrome c oxidase could explain (1) the development of different symptoms after elevated acute carboxyhemoglobin levels are normalized; (2) the observed neuropsychological effects at carbon monoxide concentrations below 5%.
Carbon monoxide can induce direct carbonylation of proline, threonine, lysine, and arginine or indirect carbonylation of cysteine groups through a lipid peroxide-dependent process.
Because oxidative stress has a critical role in CO-induced neuronal cell death, preventing its occurrence during reoxygenation is essential for a positive, neurologically protective therapeutic outcome for patients exposed to CO (03). Carbon monoxide induces the generation of reactive oxygen species resulting in lipid peroxidation and a decrease in glutathione via three different mechanisms: (1) from mitochondria during the first minutes of CO exposure; (2) from xanthine oxidase at around 20 min exposure due to energy deprivation; and (3) from NADPH oxidase in the post CO exposure period (re-oxygenation). Inhibition of these different phases with mitochondrial antioxidants, inhibitors of xanthine oxidase, or NADPH oxidase, protected neurons and astrocytes against CO-induced oxidative stress and cell death.
Pathogenesis of delayed carbon monoxide neurotoxicity. Delayed carbon monoxide neurotoxicity is complex and likely involves multiple cellular and tissue processes.
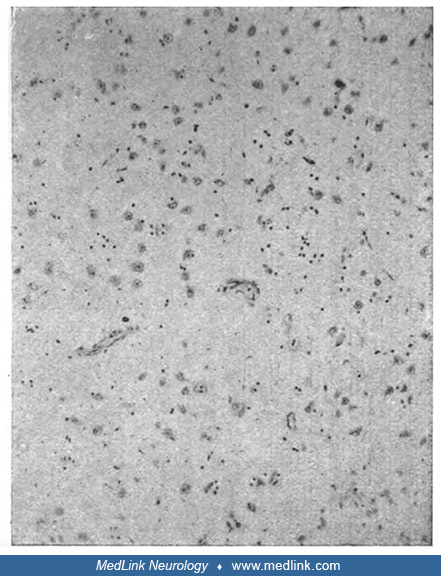
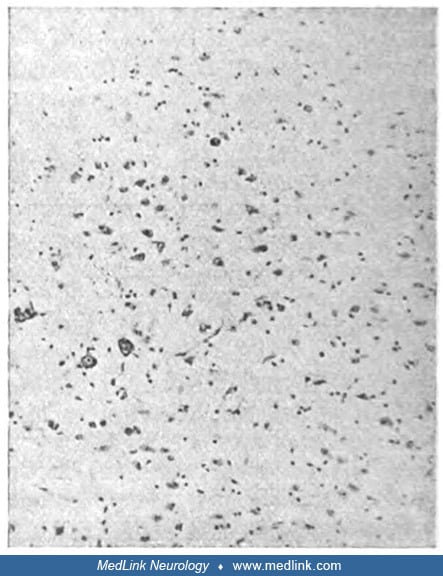
Neuropathologic manifestations of carbon monoxide poisoning include frontal lobe atrophy, diffuse demyelination of the white matter, and softening in the globus pallidus. Bilateral necrosis of the globus pallidus is a pathologic hallmark of carbon monoxide intoxication, but the presence of diffuse white matter disease is often a more reliable index of clinical status and outcome. Carbon monoxide is one of the classic causes of anoxic leukoencephalopathy (also called "delayed post-anoxic demyelination"). White matter damage is a significant factor in the pathogenesis of parkinsonism. Other affected areas include the cerebral cortex, hippocampus, cerebellum, and substantia nigra. Gray matter lesions in the cerebral cortex may include spongy changes, capillary proliferation, degeneration, and dropout of neurons. The histology of these lesions resembles the histology of lesions caused by other anoxic conditions. Carbon monoxide exposure can result in brain damage and delayed cognitive impairment, even in the absence of overt neuropathological lesions.
Carbon monoxide can trigger inflammatory processes in the brain resulting from lipid peroxidation and contributing to delayed neurologic sequelae (114; 115; 01; 95).
A study using diffusion tensor imaging found that selective damage to the corpus callosum was more profound in patients with delayed encephalopathy after carbon monoxide poisoning compared to patients without neurologic sequelae (25).
A clinical study on patients with carbon monoxide-related parkinsonism and age- and sex-matched controls used detailed neurologic examinations, diffusion tensor imaging, and PET imaging to assess disturbances in neurotransmitter pathways (24). Monoaminergic deficits and white matter damage in these patients correlated with the severity of parkinsonism and behavior changes. Sparing of the substantia nigra and topography of monoaminergic involvement suggest that pathophysiology in carbon monoxide-related parkinsonism differs from the other types.
Delayed amnesia induced by carbon monoxide exposure in mice may result from delayed neuronal death in the hippocampal CA1 subfield and dysfunction of the acetylcholinergic neurons in the frontal cortex (86). Studies in mice have shown that NMDA receptor or ion channel complex is involved in the mechanism of carbon monoxide-induced neurodegeneration and that glycine binding site antagonists, as well as NMDA antagonists, may have neuroprotective properties.
Delayed carbon monoxide-mediated neuropathology may be due in part to an adaptive immunological response to chemically modified myelin basic protein.
An allelic variant of rs1784594 single nucleotide polymorphism of PARK2, which is associated with Parkinson disease and other neurodegenerative disorders, is a risk factor for neuropsychological sequelae following acute carbon monoxide poisoning, particularly in females (77).
In an experimental study in rats, the overexpression of LINGO-1 increased following carbon monoxide poisoning, activating the RhoA associated ROCK2 signaling pathway, which may be an important pathophysiological mechanism underlying delayed encephalopathy after acute carbon monoxide poisoning (53).
Nitric oxide, which arises endogenously through the action of nitric oxide synthase enzymes, is a highly reactive molecule that plays important roles in the regulation of various body functions. It is a double-edged sword with beneficial as well as harmful effects. Carbon monoxide exposure initiates processes, including oxidative stress that triggers activation of neuronal nitric oxide synthase, which may aggravate carbon monoxide-mediated neuropathology.
Brain white matter edema seen in acute, severe carbon monoxide poisoning may result from hypoxia and subsequent acidosis, which increases vascular permeability.
Other roles for carbon monoxide. Endogenously formed carbon monoxide arises from the enzymatic degradation of heme oxygenase to release a molecule of carbon monoxide, which acts as a neuromodulator. Carbon monoxide, as an enzymatic byproduct of heme oxygenase, may be responsible for many biological functions of heme oxygenase, including cytoprotection against oxidative stress, vasodilation, neurotransmission in the central or peripheral nervous systems, and anti-inflammatory, anti-apoptotic, or anti-proliferative functions (59). Heme oxygenase-derived carbon monoxide may also act as an oxygen sensor and circadian modulator of heme biosynthesis. Some signaling effects of carbon monoxide depend on the activation of soluble guanylate cyclase or modulation of mitogen-activated protein kinase (MAPK) signaling pathways.
|
• Carbon monoxide is the leading cause of death by poisoning in the United States, accounting for more than 4000 annual deaths. |
Carbon monoxide is the leading cause of death by poisoning in the United States. More than 4000 persons die annually from carbon monoxide poisoning. In addition, carbon monoxide accounts for more than half of the approximately 12,000 annual fire-associated deaths. Approximately 50,000 emergency department visits for carbon monoxide poisoning in the USA occur annually (121). In Korea, the reported incidence of carbon monoxide poisoning in households using charcoal briquettes for heating and cooking varies from 4% to 7% in major cities. No figures are available for a much larger number of sufferers from occult carbon monoxide poisoning.
In a pilot surveillance system for unintentional, non-fire-related carbon monoxide poisoning in Maine from 1999 through 2003--utilizing hospital discharges, emergency department and hospital outpatient visits, and mortality data--740 cases were identified; 6% were hospitalized, 60% were seen in an emergency department, and 34% were seen in another outpatient setting (37). More cases were observed in fall and winter; 23% of patients aged 16 or older were classified as exposed in an occupational setting. Among disaster-related cases, more were older (at least 65 years old; 12% vs. 4%) and female (62% vs. 45%), and fewer were in occupational settings (2% vs. 23%). During the study, three separate occupational exposure incidents resulted in multiple people seeking medical care for carbon monoxide poisoning. The events in July 1999 (17 records) and January 2001 (29 records) are evident as unusually high peaks in the time series of outpatient visits for carbon monoxide poisoning by month. These two events were caused by malfunctioning forklifts (one each in an agricultural and industrial setting). The third event (seven records) in July 2003 occurred in a restaurant kitchen.
The line shows the 3-month moving average. Data include records of visits to any nonfederal hospital facility in Maine, including records of all outpatient and emergency department visits billed through a nonfederal hospital in...
Unintentional carbon monoxide poisoning. A Centers for Disease Control and Prevention (CDC) surveillance report of unintentional carbon monoxide poisoning across multiple data sources in the United States for the period 2005 to 2018 identified 39.5 poison center exposure calls per million population (nationally), 56.5 emergency department visits per million population (in 17 states), 7.3 hospitalizations per million population (in 26 states), and 3.3 deaths per million population (nationally) (104). Over this period, there was a decrease in the crude rate for non-fire-related carbon monoxide poisonings from hospital and death data. Non-fire-related cases comprised 74% of emergency department visits data, 60% of hospitalizations, and 41% of deaths compared with other unintentional causes. Across all data sources, unintentional carbon monoxide poisonings were most often reported during the winter season, notably in January and December. Children aged 0 to 9 years old had the highest reported rates in poison center exposure case data and emergency visits (54 and 71 per million population, respectively). Adults older than 80 years had the highest rates of hospitalization and deaths (20 and 10 per million, respectively). Deaths occurred more often among men and in the Midwest region. Poison center exposure call data revealed that 46% were treated at a health care facility. Headaches, nausea, and dizziness and vertigo were the most reported symptoms.
Cases in children. In a Turkish study of 380 children diagnosed with carbon monoxide poisoning between January 2017 and January 2021 and 380 healthy controls, carbon monoxide poisoning was diagnosed based on the medical history and a carboxyhemoglobin (COHb) level of more than 5% (39). The patients were classified as mild (COHb 10%), moderate (COHb 10%–25%), or severe (COHb > 25%). The most common place of exposure was at home, and all cases were affected accidentally. The coal stove was the most common source of exposure, followed by natural gas. The most common symptoms were nausea and vomiting, vertigo, and headache. Severe neurologic symptoms (eg, syncope, confusion, dyspnea, and seizures) were more common in the severe group. A total of 91% had hyperbaric oxygen therapy, 4% were intubated, and 4% were transferred to intensive care, but no deaths or sequelae were observed. COHb levels were associated with elevated troponin and lactate levels in the severe group.
Cold weather. Carbon monoxide poisoning is more common with cold weather. In a Chinese study of hospital admissions due to carbon monoxide poisoning in Guangdong, China, from 2013 to 2020, low temperature was associated with a high risk of carbon monoxide poisoning, with a lag lasting 7 days (124). In a Chinese study of emergency call data, cold waves were associated with an increased risk of CO poisoning, and the risk increased with lower temperature thresholds and longer cold wave durations (123).
In a study of nonoccupational carbon monoxide poisoning caused by household coal heating in Jinan, China, between 2007 and 2021, 5616 cases and 180 deaths were identified (76). The cumulative incidence rate was 5.8 per 100,000 person-years, and the mortality rate was 0.2 per 100,000 person-years. The incidence in urban areas (6.6 per 100,000 person-years) was significantly higher than rural areas (5.0 per 100,000 person-years). Incidence density and mortality declined over the study interval, and these were not related to changes in average temperature. Incidence density and mortality were highest during the winter months (December, January, and February). Poisoning mainly occurred during sleep hours. Socioeconomic status, accessibility to health services, and rural status were determinants for carbon monoxide poisoning incidence and mortality.
(Source: Li M, Shan B, Peng X, Chang H, Cui L. An urgent health problem of indoor air pollution: results from a 15-years carbon monoxide poisoning observed study in Jinan City. Sci Rep 2023;13[1]:1619. Creative Commons Attribut...
(Source: Li M, Shan B, Peng X, Chang H, Cui L. An urgent health problem of indoor air pollution: results from a 15-years carbon monoxide poisoning observed study in Jinan City. Sci Rep 2023;13[1]:1619. Creative Commons Attribut...
(Source: Li M, Shan B, Peng X, Chang H, Cui L. An urgent health problem of indoor air pollution: results from a 15-years carbon monoxide poisoning observed study in Jinan City. Sci Rep 2023;13[1]:1619. Creative Commons Attribut...
(Source: Li M, Shan B, Peng X, Chang H, Cui L. An urgent health problem of indoor air pollution: results from a 15-years carbon monoxide poisoning observed study in Jinan City. Sci Rep 2023;13[1]:1619. Creative Commons Attribut...
(Source: Li M, Shan B, Peng X, Chang H, Cui L. An urgent health problem of indoor air pollution: results from a 15-years carbon monoxide poisoning observed study in Jinan City. Sci Rep 2023;13[1]:1619. Creative Commons Attribut...
(Source: Li M, Shan B, Peng X, Chang H, Cui L. An urgent health problem of indoor air pollution: results from a 15-years carbon monoxide poisoning observed study in Jinan City. Sci Rep 2023;13[1]:1619. Creative Commons Attribut...
Intentional carbon monoxide poisoning (suicide). In a study of intentional carbon monoxide poisoning hospitalization rates in England between 2002 and 2016, using NHS Digital's Hospital Episode Statistics (HES) data, there were 178 hospital admissions for intentional CO poisoning per year, on average (99). The age-standardized rates decreased substantially over the study period, particularly among males (average annual percent change of -7.8%, compared to 3.9% among females), although hospital admission rates for intentional carbon monoxide poisoning were consistently much higher among males than females. Most admissions (81%) occurred in males, particularly among middle-aged men, although a secondary peak occurred among men aged 80 years and older. White men aged 35 to 44 years were particularly at risk. For the elderly, a stronger level of dependency, deterioration of mental and physical health, and social isolation may contribute to the increase in carbon monoxide suicide attempts among elderly men. Carbon monoxide poisoning hospitalization rates varied by quintiles of the Carstairs deprivation index (21), with the lowest male rates in the most deprived group (99). Limited access to vehicles by highly deprived individuals may explain the lower rates of intentional carbon monoxide poisoning across the most deprived; in any case, intentional carbon monoxide poisoning from automobile exhaust has been much less common since the introduction of catalytic converters in the 1990s.
The scale is presented on the left y-axis. Shaded areas indicate 95% confidence intervals. Dashed lines present the age-standardized suicide rate, regardless of method, by gender in England with the corresponding scale on the r...
Bars show the age-standardized rates (left y-axis). Dashed lines reflect number of cases (right y-axis). Counts under 10 have been suppressed, hence, the interrupted lines. Population standardized to the 2013 European Standard ...
Rates per 100,000 inhabitants and age-standardized to the 2013 European Standard Population. Error bars show the 95% confidence intervals. The Carstairs Index is a composite measure of unemployment, household overcrowding, car ...
Suicide by charcoal burning has been an increasingly common suicide method in Hong Kong since first reported in 1998, and it has spread into South Korea, Taiwan, Japan, and other countries. In a systematic scoping review of 88 studies, most from East Asia, charcoal burning suicide rates in Hong Kong, Taiwan, and Japan have passed their peaks, whereas rates are continuing to increase in South Korea (125). Restricting access to charcoal and raising public awareness have been effective in the short term in preventing charcoal burning suicide.
Risk factors for delayed encephalopathy. The following risk factors have been identified for the development of delayed encephalopathy following carbon monoxide poisoning: (1) old age; (2) hypertension; (3) prolonged coma lasting 2 to 3 days; (4) persisting dizziness and fatigue after regaining consciousness; and (5) excessive mental stimulation during recovery.
A retrospective study of case records of patients with carbon monoxide poisoning found that the Glasgow Coma Scale score, MMSE scores, and positive findings in brain CT scans were predictors of the development of delayed neuropsychological sequelae, but carboxyhemoglobin level was not (64).
Another retrospective study identified several predictors of delayed neuropsychological sequelae after carbon monoxide poisoning that can be assessed in the emergency department: carbon monoxide exposure duration of more than 6 hours, a Glasgow Coma Scale score less than 9, seizures, systolic blood pressure lower than 90 mmHg, elevated creatine phosphokinase concentration, and leukocytosis (92).
|
• Prevention is the most important aspect of the management of carbon monoxide poisoning. | |
|
• Various preventive measures should include control of atmospheric pollution (mainly from automobile exhaust), proper maintenance of household gas appliances, and elimination of indoor charcoal and wood burning without proper exhaust. |
Prevention is the most important aspect of management of carbon monoxide poisoning. Important preventive measures include: (1) control of atmospheric pollution (mainly from automobile exhaust); (2) occupational measures to limit exposure in at-risk workers (eg, establishment and enforcement of safety standards; protective equipment; monitoring of carbon monoxide levels in the working environment and in individual at-risk workers); (3) residential measures to prevent carbon monoxide exposures (eg, proper maintenance of household gas appliances, elimination of indoor charcoal and wood burning without proper exhaust, and monitoring of carbon monoxide levels); (4) personal measures (eg, smoking cessation and avoidance of second-hand smoke).
Tobacco smoking. The average carboxyhemoglobin in chronic, heavy smokers is approximately 5%. This puts smokers at greater risk of carbon monoxide poisoning when exposed to low concentrations of environmental carbon monoxide that may not produce symptoms in nonsmokers. The increased incidence of atherosclerotic disease in smokers has been attributed to exposure to carbon monoxide (rather than nicotine or other toxins in cigarette smoke), but the role of chronic carbon monoxide exposure in the development of atherosclerotic disease is unproven.
Canaries (and mice) in coal mines. Scottish physiologist John Scott Haldane demonstrated that most British miners did not die of lack of oxygen or trauma after colliery (ie, coal mine) accidents but of carbon monoxide poisoning. To judge the safety of mine air, the miners had naively relied on the ability of their candle or lamp to burn, but the presence of carbon monoxide would not influence this. He suggested that canaries could serve as sentinel animals, effectively biosensors, which due to their small size and correspondingly relatively higher metabolism, would lose consciousness about 20 minutes before humans. Canaries were effectively used to identify carbon monoxide in mines from the early 1910s to the mid-1980s (05; 17; 17; 18; 19; 108; 32).
|
There, is, however, another method, which would, I feel confident, be practically successful. In small animals, the rate at which the blood becomes saturated with carbon monoxide is far more rapid than in man; hence, a small animal, such as a mouse, shows the effects of the gas far more rapidly than a man, although a given percentage of it seems not to be, in the long run, more poisonous to a mouse than to a man. Practically speaking, the condition of a mouse that has been for a very short time in a poisonous percentage of carbon monoxide, indicates what will be the condition of a man carrying it after a much more prolonged stay in the same atmosphere. With a man at rest it takes about 20 times as long for the man as for the mouse to be distinctly affected by the gas. Thus, to take an example, I found that with .4%, a mouse was distinctly affected in one and a half minutes, and quite helpless in 3 minutes, while I myself was not distinctly affected until after half an hour. The air I was breathing contained about the same percentage as is so often fatal to rescuers. These experiments show distinctly how valuable the indications given by a mouse, or other small animal, would be to men exposed to danger from after-damp. The mouse may be carried in a small cage, or a lamp chimney closed at the ends with wire gauze. When dangerous percentages of carbon monoxide are encountered the mouse will begin to pant and show signs of weakness in the legs; should the mouse suddenly become unconscious, danger is imminent. A few white mice might easily be kept in the engine room of the winding engine, or in stables or other places in the pit (47). |
A relief is a wall-mounted sculpture in which the 3-dimensional elements are raised from a flat base. Location: Building, 16 rue du 22-Novembre, Strasbourg, France. The corridor-vestibule, the stairwell with its stained glass w...
Chemical detectors. Demand for carbon monoxide detectors initially came from industry. By the early 1920s, safety monitors were being sold to improve industrial safety, including for use in mines. In 1921, GH Burrell, brother of George A Burrell, the inventor of the charcoal gasoline extraction process, invented a “very sensitive carbon monoxide detector that combined a syringe for sampling air and a container of a proprietary chemical that changed color in the presence of carbon monoxide (102). Burrell first used his detector in a steel mill office, where the staff complained about headaches. Using the detector at multiple sites around the building, he discovered that an underground flue was not venting properly.
In 1925, Chester S Gordon and James T Lowe of the American Telephone and Telegraph Company patented a carbon monoxide detector for use in confined spaces, like manholes, where gases could collect (patent US1644014A); their detector combined a reagent (palladium chloride) and a carrier solution in a glass vial covered with absorbent cotton.
This carbon monoxide detector was invented in 1925 by Chester S Gordon and James T Lowe of the American Telephone and Telegraph Company (patent US1644014A). The detector combined a reagent (palladium chloride) and a carrier sol...
To test for carbon monoxide, a user crushed the glass, allowed the reagent solution to saturate the cotton, and watched to see if the covering grew dark. Unfortunately, these early sensors were only semiquantitative (based on the degree of color change).
Industry demand for more complex sensors increased in the 1970s and 1980s, resulting in biomimetic, electrochemical, and semiconductor sensors.
Battery-powered home carbon monoxide detectors followed the popularity of battery-powered smoke alarms; the first such monofunctional devices were available in 1993, followed by a combined carbon monoxide and smoke detector in 1996, and a remote-controlled version in 2000.
Modern semiconductor carbon monoxide sensors consist of two tin dioxide wires on a ceramic base in an integrated circuit. The sensing element must be heated to 400 degrees C to operate. A change in resistivity indicates the presence of carbon monoxide. Because of the power requirements for this technology, it is usually powered by a building’s electrical system rather than batteries: hardwired versions can last up to 10 years, whereas battery-powered versions last only a few months.
Newer models often include a feature that will send an alarm message to a smartphone, along with a sound or voice alert in the building where the detector is installed. Some models connect to Z-Wave-compatible hubs to allow syncing with other smart devices; others are compatible with Amazon’s Alexa and Samsung’s SmartThings.
Levels of carbon monoxide in the bloodstream can be tested with a “blower” device like that used to detect alcohol levels.
Standards and laws. In the United States, carbon monoxide alarms must conform to the Underwriters Laboratories 2034 standard, which is based on a threshold carboxyhemoglobin in the blood as a biomarker for time to alarm. Underwriters Laboratories has a separate standard (2075) for what they define as "detectors" --passive indicators that show the presence, absence, and level of carbon monoxide.
States have responsibility for providing laws and regulations concerning carbon monoxide detectors. As of 2022, with few exceptions, states require the use of carbon monoxide detectors in homes, although some of these limit the requirement to new construction or transfer of ownership; the exceptions are Hawaii, Kansas, and Missouri. A few states require the detectors in hotels (at least 15 states) and schools (at least five states).
Approximately one third of nonfatal cases of carbon monoxide poisoning go undetected and undiagnosed. Carbon monoxide poisoning mimics several other diseases and is frequently misdiagnosed as psychiatric illness, migraine headaches, stroke, epilepsy, dementia, alcohol intoxication, flu-like illness, gastroenteritis, pneumonia, and heart disease. Differential diagnosis from psychiatric disorders is sometimes difficult, as the patients who attempt suicide with carbon monoxide may have had psychiatric disorders prior to carbon monoxide poisoning, and some behavioral problems may be late sequelae of carbon monoxide poisoning. Carbon monoxide poisoning may mimic decompression syndrome in divers exposed to air contamination from exhausts of nearby gasoline engines. Another frequent misdiagnosis is food poisoning. Symptoms in other household members and pets, recurring at a certain time and place, and related to the use of a gas heater, a vehicle, or any other potential source of carbon monoxide, should suggest the diagnosis. A bit of detective work may be required to locate the source of carbon monoxide poisoning. Diagnosis of carbon monoxide poisoning is particularly difficult in infants without a history of exposure, particularly because convulsions and altered states of consciousness are manifestations of many other illnesses in infants.
The CH2OPD2 mnemonic (Community, Home, Hobbies, Occupation, Personal habits, Diet, and Drugs) is helpful in obtaining an environmental exposure history.
Occult carbon monoxide poisoning should be considered in the differential diagnosis of neurologic disorders; if any suspicion exists, carboxyhemoglobin levels should be checked. Response to oxygen or hyperbaric oxygen supports the diagnosis of acute carbon monoxide poisoning, but the lack of response does not exclude it. Characteristic CT lesions are helpful in diagnosis. Akinetic mutism following carbon monoxide poisoning results from injury to frontal-subcortical circuits and is distinct from locked-in syndrome.
|
• The most important diagnostic test for carbon monoxide poisoning is the direct spectroscopic measurement of carboxyhemoglobin levels in the blood. | |
|
• Carboxyhemoglobin levels greater than 10% have diagnostic significance, although carboxyhemoglobin levels do not necessarily correspond to the severity of the clinical symptoms. | |
|
• CT and MRI are valuable in delineating disease extent and different patterns of brain injury in the acute and delayed stages of carbon monoxide poisoning. |
The 2019 Centers for Disease Control and Prevention case definition of carbon monoxide poisoning. The Centers for Disease Control and Prevention has established a case definition for carbon monoxide poisoning (22).
|
Clinical criteria | ||
|
Presumptive clinical evidence | ||
|
• Loss of consciousness OR | ||
|
• Death | ||
|
Supportive clinical evidence | ||
|
• A person with signs or symptoms consistent with carbon monoxide poisoning, which may include elevated pulse CO-oximetry measurement or nonspecific symptoms (eg, nausea, vomiting, confusion, shortness of breath, and chest pain) | ||
|
Laboratory criteria | ||
|
Confirmatory laboratory evidence | ||
|
• A person who does not smoke, or a child (age < 14 years) whose smoking status is unknown and has a carboxyhemoglobin level of ≥ 5.0% as measured in a blood sample OR | ||
|
• A person who smokes, or a person (age ≥ 14 years) whose smoking status is unknown, with a carboxyhemoglobin level of > 12.0% as measured in a blood sample | ||
|
Presumptive laboratory evidence | ||
|
• A person who smokes, or whose smoking status is unknown (age ≥ 14 years) and has a carboxyhemoglobin (COHb) level of ≥ 9.0% and ≤ 12.0% as measured in a blood sample | ||
|
Supportive laboratory evidence | ||
|
• A person who does not smoke, or a child (age < 14 years) whose smoking status is unknown and has a carboxyhemoglobin (COHb) level of ≥ 2.5% and < 5.0% as measured in a blood sample OR | ||
|
• A person who smokes, or whose smoking status is unknown (age ≥ 14 years) and has a carboxyhemoglobin (COHb) level of ≥ 7.0% and < 9.0% as measured in a blood sample | ||
|
Case classification | ||
|
Suspected | ||
|
• A person with supportive laboratory evidence OR | ||
|
• A person with supportive clinical criteria AND possible exposure evidence | ||
|
Probable | ||
|
• A person with presumptive laboratory evidence* OR | ||
|
• A person with presumptive clinical evidence AND possible exposure evidence OR | ||
|
• A person with presumptive or supportive clinical evidence AND epidemiologic linkage | ||
|
Confirmed | ||
|
• A person with confirmatory laboratory evidence* OR | ||
|
• A person with presumptive or supportive clinical evidence AND with confirmatory exposure evidence | ||
|
*Other plausible clinical explanations should be considered, including chronic obstructive lung disease and hemolysis. | ||
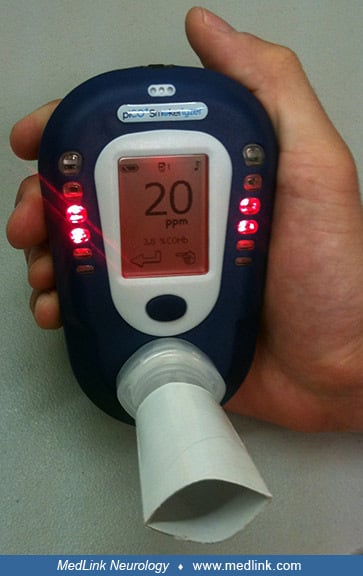
Laboratory studies. The most important diagnostic test for carbon monoxide poisoning is the direct spectroscopic measurement of carboxyhemoglobin levels in the blood. This can be measured rapidly with a fingertip carboxyhemoglobin saturation monitor. An indirect way to estimate carbon monoxide exposure or poisoning is to measure the carbon monoxide content of exhaled breath.
Emergency medical technicians can effectively screen for elevated carbon monoxide levels during emergency responses using handheld carbon monoxide meters that detect as little as one part per million of carbon monoxide. In severely ill patients with suspected exposure to carbon monoxide, oxygen therapy should not be delayed pending carboxyhemoglobin estimation, even though oxygen, by lowering carboxyhemoglobin, may remove the evidence of carbon monoxide exposure. Carboxyhemoglobin levels greater than 10% have diagnostic significance, although carboxyhemoglobin levels do not necessarily correspond to the severity of the clinical symptoms.
|
Carboxyhemoglobin |
Interpretation |
|
Less than 5% |
normal |
|
5% to 10% |
normal in a smoker |
|
Greater than 10% |
abnormal, consider high-flow oxygen |
|
Greater than 15% |
significantly abnormal, requires treatment |
Blood samples should also be used for determining blood gases, blood counts, cardiac enzymes, and basic biochemical investigations. The patient should be screened for other drugs and alcohol intoxication, and in the case of women of childbearing age, a pregnancy test should be done. Various other tests are selected according to the organs affected, severity of the poisoning, and the facilities available. An electrocardiogram can detect cardiac arrhythmias, and a chest x-ray can detect pulmonary edema and aspiration pneumonia, particularly in a comatose patient.
Electrophysiology. ECG should be done to assess for evidence of myocardial ischemia, abnormal rhythm, or conduction disturbance.
Electroencephalography abnormalities are mostly diffuse and include continuous theta and delta activity, rhythmic bisynchronous bursts of slow waves, low-voltage activity with or without periods of silence or spiking, burst suppression pattern, and electrocerebral silence.
Topographic quantitative EEG methods may have promise in the study of acute and long-term effects of carbon monoxide poisoning. Quantitative electroencephalography has been used to monitor the efficacy of hyperbaric oxygen treatment of carbon monoxide poisoning, and improvement of occipital alpha activity is a possible indicator of treatment efficacy.
Assessment for concomitant poisoning. If the cause of carbon monoxide poisoning is not obviously accidental, concomitant poisoning should be considered (eg, alcohol, aspirin, acetaminophen); urine toxicology, as well as salicylate and acetaminophen levels, should be obtained. In cases of suspected or documented carbon monoxide poisoning due to fires or smoke inhalation, concomitant cyanide poisoning should be considered (58; 74; 06; 84).
Brain imaging studies. CT and MRI are valuable in delineating disease extent and different patterns of brain injury in the acute and delayed stages of carbon monoxide poisoning.
CT is the most widely used neuroimaging method for patients with carbon monoxide poisoning. Common CT findings are symmetrical low-density basal ganglia abnormalities and diffuse low-density lesions of the white matter.
Lucencies or other abnormalities in the globi pallidi may be unilateral, and white matter involvement may also show marked asymmetry. Postcontrast CT offers an advantage when noncontrast CT is normal in carbon monoxide poisoning. In the so-called "interval form" of carbon monoxide poisoning (ie, with a lucid interval between the acute phase of intoxication and emergence of delayed neurologic sequelae), low-density lesions bilaterally in the frontal regions, centrum semiovale, and globi pallidi are associated with white matter demyelination of the corresponding parts at autopsy. CT and MRI have demonstrated hemorrhagic infarction in the white matter following acute carbon monoxide poisoning. An initial normal CT scan in a comatose patient does not rule out carbon monoxide poisoning.
MRI studies in the acute phase of carbon monoxide poisoning show that, although the globus pallidus is the commonest site of abnormality in the brain, the effects on the brain are widespread. In a Chinese study of 41 patients with carbon monoxide poisoning (24 patients with delayed neurologic sequelae) and 36 sex- and age-matched healthy controls, gray matter atrophy in the medial orbital superior frontal gyrus, anterior cingulate cortex, thalamus, hippocampus, and posterior cerebellum was associated with early neurologic deficits in patients with carbon monoxide poisoning (127). Greater atrophy occurred in patients with delayed neurologic sequelae and those with T2 hyperintense lesions.
The white matter hyperintensities seen on MRI do not correlate with carbon monoxide poisoning severity except those in the centrum semiovale, which are significantly associated with cognitive impairments. Diffusion-weighted MRI is useful for early identification of pallidoreticular damage due to acute carbon monoxide poisoning.
The combination of proton magnetic resonance spectroscopy and diffusion tensor imaging on a 3.0T system is useful for monitoring the changes in brain damage and the clinical symptoms of patients with delayed encephalopathy after carbon monoxide poisoning and response to hyperbaric oxygen treatment (113).
Predictors of late sequelae. Several potential predictors of delayed neurologic sequelae following carbon monoxide poisoning have been identified, including Glasgow Coma Scale less than 9, Glasgow–Pittsburgh cerebral performance category (56; 57), transient loss of consciousness, longer duration from carbon monoxide exposure to presentation in the emergency department, corrected QT (QTc) prolongation, the bispectral index (BIS; a measure of the level of consciousness by algorithmic analysis of a patient's electroencephalogram, usually applied to assess the depth of anesthesia), and abnormalities on diffusion-weighted MRI (61; 78; 126).
Ideally, an objective biochemical marker could help evaluate carbon monoxide poisoning and establish a clear indication for hyperbaric oxygen therapy, but most of the studied measures remain primarily research tools. Some studies found statistical differences between carbon-monoxide-poisoned patients and controls in the frequency of delayed neurologic outcomes, but none of these tests are sufficiently useful for guiding therapy or predicting outcomes to warrant general use.
Serum markers studied include (1) tau; (2) S100β protein (a calcium-binding peptide that is used as a parameter of glial activation or death); (3) neuron-specific enolase (an isoenzyme of the glycolytic enzyme enolase and a biomarker of neuronal cell damage); and (4) C-terminal hydrolase-L1 (a marker of neuronal damage after acute neurologic insults) (91; 36; 90; 126). Serum levels of both tau and S100β proteins are elevated in patients with carbon monoxide poisoning and loss of consciousness, but tau is a more sensitive biomarker than S100β in the early stage of neurotoxic effects (36). Serum neuron-specific enolase may augment the predictive value of the initial Glasgow Coma Scale score for predicting the development of late neurologic sequelae (23).
CSF markers studied include (1) S100β protein and (2) myelin basic protein (55). Even when serum S100β protein levels are not elevated, elevated CSF S100β levels within 24 hours after carbon monoxide exposure are associated with an increased risk of developing a persistent vegetative state or delayed encephalopathy (55).
Based on current evidence, MRI may be useful in identifying delayed neurologic sequelae caused by acute CO poisoning. In a systematic review and meta-analysis of six studies comprising 635 participants, the pooled sensitivity was 0.72 (95% CI: 0.62–0.81), and the pooled specificity was 0.80 (95% CI: 0.71–0.86) (34).
Fractional anisotropy from diffusion tensor imaging in the centrum semiovale can provide a quantitative indicator of the extent of demyelination in damaged cerebral white matter during the subacute phase of carbon monoxide poisoning (10).
|
• The objectives of treatment of carbon monoxide poisoning are to hasten the elimination of carbon monoxide from the body and to counteract hypoxia and direct tissue toxicity of carbon monoxide. | |
|
• The mainstays of therapy for carbon monoxide poisoning are supplemental oxygen, ventilatory support, and monitoring for cardiac dysrhythmias. | |
|
• Adequate treatment of carbon monoxide poisoning in the acute stage with hyperbaric oxygen may reduce late neurologic sequelae. | |
|
• Although hyperbaric oxygen therapy has been used as a treatment for carbon monoxide poisoning since 1960, specific indications for its use have remained controversial. |
The supreme objectives in the treatment of carbon monoxide poisoning are to correct tissue hypoxia and remove carbon monoxide to avert acute and long-term sequelae. Therefore, the mainstays of therapy are to administer supplemental oxygen, provide ventilatory support, and monitor for cardiac dysrhythmias. Although immediate oxygen breathing is sometimes an adequate treatment, hyperbaric oxygen therapy is favored.
General measures. The first step is to remove the patient from the exposure site and immediately administer 100% oxygen by a non-rebreather mask. Comatose patients require endotracheal intubation followed by 100% oxygen. Other complications may necessitate the use of additional medications, for example, mannitol and corticosteroids for cerebral edema, diuretics for pulmonary edema, antiarrhythmic drugs for cardiac arrhythmias, antibiotics for pneumonia, and sodium bicarbonate to correct acidosis.
The severity of carbon monoxide poisoning influences the management strategy. Mild poisoning (carboxyhemoglobin less than 30%) may be managed symptomatically, and 100% oxygen is administered until the carboxyhemoglobin level drops to below 5%. Such patients may be discharged home from the emergency department; admission should be considered if neurologic or cardiac function is impaired.
Patients with smoke inhalation and suspected carbon monoxide and concomitant cyanide poisoning should also be treated with a hydroxocobalamin kit or an emergency cyanide kit (note that hydroxocobalamin can interfere with the measurement of carboxyhemoglobin).
For the treatment of carbon monoxide-induced headaches, oxygen inhalation alone is as effective as oxygen plus metoclopramide or oxygen plus metamizole sodium (88).
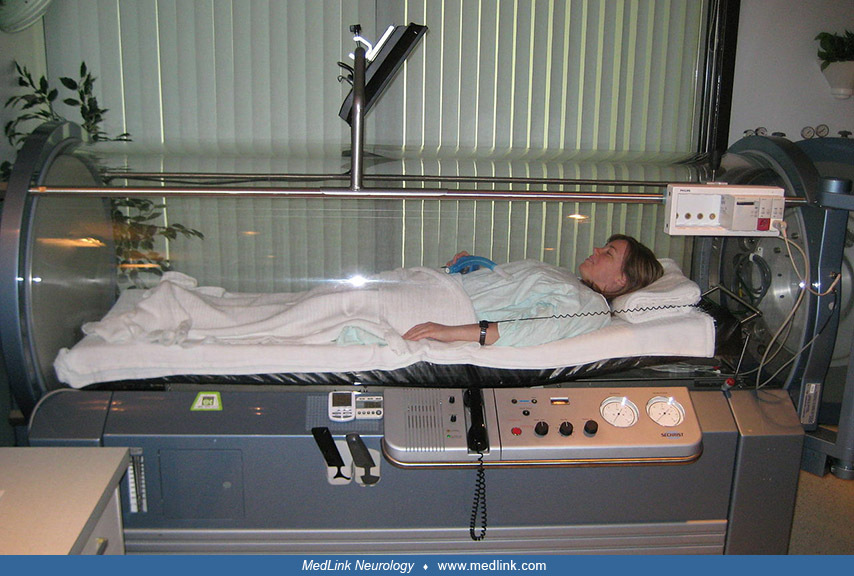
Hyperbaric oxygen therapy. Hyperbaric oxygen therapy involves the administration of 100% oxygen at pressures greater than that at sea level.
At 3 atmospheres absolute pressure (ATA), oxygen dissolves in the plasma with a partial pressure of oxygen of 2193 mm Hg, which suffices to sustain life even in the complete absence of functional hemoglobin. Therefore, hyperbaric oxygen therapy can counteract tissue hypoxia despite the presence of high levels of carboxyhemoglobin and a corresponding marked reduction of functional hemoglobin. Additionally, hyperbaric oxygen therapy causes a rapid reduction of carbon monoxide in the blood by mass action of oxygen. The half-life of carbon monoxide when breathing air is 5 hours 20 minutes, but this can be reduced to 1 hour 20 minutes when breathing 100% oxygen at normal atmospheric pressure and to 23 minutes during hyperbaric oxygen therapy. However, hyperbaric oxygen therapy may not reverse tissue injury that has already occurred, and it may not halt adverse cellular and tissue processes acting through other mechanisms (eg, disruption of cellular respiration by prior binding to cytochromes).
Although hyperbaric oxygen therapy has been used as a treatment for carbon monoxide poisoning since 1960, specific indications for its use have remained controversial. Earlier conclusions about efficacy were based on clinical experience and uncontrolled studies, but multiple randomized clinical trials and meta-analyses have been reported comparing hyperbaric oxygen therapy and normobaric oxygen administration (94; 29; 116; 103; 122; 48; 51; 04; 16; 35; 79; 119). The most recent meta-analyses are summarized below.
In a Cochrane review, six randomized controlled trials of varying quality were identified that evaluated clinical outcomes (16). Design or analysis flaws were evident in all the trials. Among the six trials involving 1361 participants, two found a beneficial effect of hyperbaric oxygen therapy for the reduction of neurologic sequelae at 1 month, while four others did not (although three of these had low power to detect a benefit). A pooled random effects meta-analysis did not suggest a significant benefit from hyperbaric oxygen therapy, but the interpretation was complicated by significant methodologic and statistical heterogeneity among the trials. The authors concluded that existing randomized trials do not establish whether hyperbaric oxygen therapy reduces the incidence of adverse neurologic outcomes in patients with carbon monoxide poisoning. Additional research is needed to better define the role, if any, of hyperbaric oxygen therapy in the treatment of patients with carbon monoxide poisoning.
A later meta-analysis of trials of hyperbaric oxygen therapy in patients with carbon monoxide poisoning concluded that, compared with normobaric oxygen treatment, hyperbaric oxygen therapy results in a lower incidence of neuropsychological sequelae, including headache, memory impairment, difficulty concentrating, disturbed sleep, and delayed neurologic sequelae (79). However, despite these strong claims, none of the comparisons were statistically significant.
The most recent meta-analysis of trials of hyperbaric oxygen therapy in patients with carbon monoxide poisoning included seven randomized controlled trials with a total of 2023 patients with carbon monoxide poisoning. The authors reported that, compared with normobaric oxygen treatment, a single session of hyperbaric oxygen therapy was associated with a lower risk of memory impairment. In contrast, two sessions of hyperbaric oxygen therapy were associated with a higher risk of memory impairment than one session. It is unclear whether the worse outcomes with two sessions of hyperbaric oxygen therapy reflect differences in case selection or an adverse effect of hyperbaric oxygen therapy. Hyperbaric oxygen therapy was also associated with increased block design and trail-making test scores when compared with normobaric oxygen treatment. No other significant differences were observed in those who received hyperbaric oxygen therapy versus normobaric oxygen. These results are hardly compelling and do not suggest a marked benefit of hyperbaric oxygen therapy. However, positive results may be diluted by the inclusion of cases unlikely to respond or benefit from hyperbaric oxygen therapy, and refinement of indications and optimization of hyperbaric oxygen therapy protocols may result in better outcomes.
Despite the collectively questionable benefits of hyperbaric oxygen therapy in available trials, advocates nevertheless recommend hyperbaric oxygen therapy globally for carbon monoxide poisoning or in restricted groups of patients. Often, however, such advocates cherry-pick results in support of hyperbaric oxygen therapy and provide anecdotal evidence from individual (uncontrolled) cases in support of their recommendations. Consequently, until there are better quality randomized controlled trials, the use of hyperbaric oxygen therapy for carbon monoxide poisoning is likely to remain controversial with contested indications.
Approximately 1500 carbon monoxide-poisoned patients are treated with hyperbaric oxygen therapy in the United States annually, and most treating facilities do not routinely give more than one hyperbaric oxygen therapy session. Hyperbaric oxygen therapy has also been used for the prevention and treatment of late sequelae of carbon monoxide poisoning. Indications for hyperbaric oxygen therapy for carbon monoxide poisoning are not universally recognized (85).
Advocated indications for hyperbaric oxygen therapy in carbon monoxide poisoning:
|
• Acute symptomatic carbon monoxide poisoning (122; 120; 83) | |
|
• Carboxyhemoglobin level of greater than 25% to 30%, regardless of the severity of clinical signs (83) | |
|
• End-organ ischemia (eg, chest pain, ECG changes, arrhythmias; pH less than 7.1) (83; 85; 15) | |
|
• Transient or persistent unconsciousness (83; 85; 15) | |
|
• Coma on hospital admission | |
|
• New neurologic deficits or mental status changes (85; 15) | |
|
• Persisting neurologic abnormalities | |
|
• Pregnancy (especially if the carboxyhemoglobin level is more than 15% to 20%) (83; 85; 15) | |
|
• Failure to respond to 100% oxygen after 4 to 6 hours (83) | |
|
• Recurrent symptoms up to 3 weeks after exposure (83) |
More than half of European centers initially give a single session of hyperbaric oxygen therapy, which is repeated if symptoms persist (85). Administration of more than one course of hyperbaric oxygen therapy to those with persistent coma remains controversial, even among hyperbaric oxygen therapy advocates. In practice, empirical overtreatment has often been used in the hope that it would prevent late sequelae.
Various regimens have been used to treat carbon monoxide poisoning, and there is little consistency across centers (85). The pressures used vary between 2 ATA and 3 ATA. The most frequently used protocol is an initial 45 minutes of 100% oxygen at 3 ATA followed by further treatment at 2 ATA for 2 hours or until the carboxyhemoglobin is less than 10%.
Complications of hyperbaric oxygen therapy in comatose patients include rupture of the eardrum in about 10% of the patients. Seizures may occur in patients with brain injury who are subjected to high hyperbaric oxygen pressures.
Mild hypothermia. Early mild hypothermia treatment can significantly reduce the severity of brain injury after carbon monoxide poisoning (126).
Prevention and treatment of late sequelae of carbon monoxide poisoning. The incidence and severity of secondary syndromes may be lessened by adequate treatment of carbon monoxide poisoning in the acute stage with hyperbaric oxygen therapy (116).
A randomized, prospective study found that early administration of erythropoietin to patients with carbon monoxide poisoning improved neurologic outcomes and reduced the incidence of delayed neurologic sequelae (89).
In a South Korean prospective registry-based study, early dexamethasone treatment was significantly associated with a decreased incidence of delayed neuropsychiatric sequelae (60). A higher Glasgow Coma Scale at presentation was associated with a lower incidence of delayed neuropsychiatric sequelae. A controlled trial is needed for confirmation.
Various other treatments for sequelae of carbon monoxide poisoning have been advocated in anecdotal reports, small uncontrolled case series, or low-quality comparative trials.
Endogenous production of carbon monoxide is doubled in women during the progesterone phase of the menstrual cycle compared to the level during the estrogen phase or to levels in men. During pregnancy, the rate of endogenous production of carbon monoxide increases even further, but it declines rapidly in the postpartum period.
Carbon monoxide diffuses readily across the placenta, but its elimination from the fetus lags considerably behind that of the mother. Because fetal hemoglobin has a greater affinity for carbon monoxide than hemoglobin in the mother's red blood cells, the fetus acts as a "sink" for the carbon monoxide. Congenital malformations, fetal neurotoxicity, and fetal death may result if carboxyhemoglobin levels are markedly elevated or if moderately severe maternal toxicity occurs. Carbon monoxide intoxication of the pregnant mother during the first trimester may lead to congenital malformations in the offspring. Severe carbon monoxide poisoning during the latter months of pregnancy can lead to fetal brain lesions like those seen in adults.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Douglas J Lanska MD MS MSPH
Dr. Lanska of the University of Wisconsin School of Medicine and Public Health and the Medical College of Wisconsin has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Neurobehavioral & Cognitive Disorders
Jul. 19, 2024
Peripheral Neuropathies
Jul. 17, 2024
Neuro-Ophthalmology & Neuro-Otology
Jul. 17, 2024
Neuro-Ophthalmology & Neuro-Otology
Jul. 17, 2024
General Neurology
Jul. 11, 2024
Headache & Pain
Jul. 06, 2024
Neuro-Ophthalmology & Neuro-Otology
Jun. 21, 2024
General Neurology
Jun. 11, 2024