Infectious Disorders
Zika virus: neurologic complications
Oct. 08, 2024
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Neurocysticercosis continues to be the most common CNS parasite worldwide and is becoming increasingly identified in the United States. Most patients in the United States present with seizures (focal or generalized) or headache and have come from Mexico, Latin America, or the Indian subcontinent. A new set of diagnostic criteria has been published. There are now three serological methods of diagnosing neurocysticercosis. A serum or CSF antibody assay, especially an enzyme-linked immunotransfer blot assay, is quite sensitive and specific but is not widely available, particularly in the Indian subcontinent. The utilization of next-generation sequencing on cerebrospinal fluid presents a promising approach for the accurate diagnosis and continuous monitoring of neurocysticercosis. This assay is the most valuable in diagnosing extraparenchymal neurocysticercosis in the ventricle or meninges and can be utilized to follow patient response to treatment. It appears that the cysticercus cyst releases specific proteins that induce an anti-inflammatory host response to prevent the host from destroying viable cerebral cysts. Although some controversy continues regarding the necessity of treating single neurocysticercosis cysts in developing countries, studies, including a 2013 guideline published in Neurology and a meta-analysis report, find that albendazole treatment significantly hastens the disappearance of cysts and reduces the incidence of seizures with generalization. For intraventricular neurocysticercosis, endoscopic removal is an effective and safe treatment option. Optimal management of disseminated cysticercosis is currently unclear. Increasing numbers of patients with disseminated cysticercosis are now treated with antiparasitic drugs. Experts suggest administering low doses of a single antiparasitic drug along with corticosteroids. Repeated cycles of albendazole therapy lead to a significant reduction in lesion load and improvements in seizures and headache as well. None of the patients studied had any serious side effects. Approximately 38% of parenchymal neurocysticercosis cysts become calcified even after antiparasitic treatment. Calcified lesions can be a persistent source of seizure recurrences.
|
• Neurocysticercosis is the most common CNS parasite worldwide, with most U.S. cases coming from immigrants from Mexico and Latin America. | |
|
• Neurocysticercosis comes from eating viable cysts from human feces of individuals infected with the pork tapeworm and not from eating undercooked infected pork meat. | |
|
• So long as organisms remain viable in the brain, the patient is usually asymptomatic due to the parasite cyst producing proteins that prevent the host from initiating an inflammatory response to destroy the cyst. | |
|
• Seizures are the most common clinical manifestation, and headaches are the second most common. | |
|
• Diagnosis is usually made by demonstration of typical cysts on MRI or CT scan plus presence of cysticercosis enzyme-linked immunoelectrotransfer blot antibody assay in serum or CSF. | |
|
• Treatment of parenchymal cysts using albendazole with or without corticosteroids hastens disappearance of parenchymal cysts and reduces rate of seizure recurrence, but treatment of extraparenchymal cysts in meninges or ventricles is difficult and often requires ventricular shunting and repeated courses of albendazole and long-term steroids. |
Cysticercosis is a zoonotic infection involving pigs and man and has an ancient history (34). Tapeworms have been found in 3000 B.C. Egyptian mummies. Human tapeworms were recognized by Hippocrates, and "mealy" pork containing cysticerci were described by Aristotle. Cysticercosis likely was recognized early on as dangerous to humans in that the ancient Hebrew Bible forbid consumption of pork and the ancient Koran also forbid pork consumption.
Many ex-soldiers who served in India from 1926 until 1929, after returning from India, were reported to be suffering from epilepsy. In the majority, epilepsy proved to be due to cysticercosis. The diagnosis was based on one of four criteria: palpation of subcutaneous nodules of calcified cysts; x-ray demonstration of calcified cysts in the muscles or in the skull; the presence of eosinophils in the spinal fluid, not a common finding; or a positive complement-fixation test (12; 65; 26). Praziquantel was approved for medical use in the United States in 1982. Sotelo and co-workers, in 1984, demonstrated that praziquantel is effective in cysticercosis of the brain parenchyma (129).
Neurocysticercosis is clinically a pleomorphic disease that may be asymptomatic or manifest a variety of nonspecific mild to severe neurologic syndromes. Clinical signs in neurocysticercosis depend on the number, size, and stage of cysts, CNS location, and severity of inflammatory response against the parasites (see Table 1). In general, cysticerci do not produce clinical symptomatology until the cyst begins to die. The inflammatory reaction triggered by the cyst degeneration produces clinical symptoms such as seizures, headaches, altered mental status, and focal neurologic signs like hemiparesis, visual loss, and paraparesis.
|
Signs and symptoms* |
Percentage |
|
• Seizures |
75% |
Patients may present with more than one sign. Estimated from author's personal experience and the following references (41; 134; 86; 97; 42; 122; 15; 123; 64).
Seizures, either focal or generalized, are the most common presenting complaint and occur in up to 75% of patients (32; 15; 123). Most patients have a normal neurologic examination. However, a study from Brazil reported dementia in 13% of patients (21). Longitudinal studies suggest that cognition improves with treatment (141).
Headaches are the second most common clinical manifestation. The headache has several causes and may come from intracranial hypertension. The intracranial hypertension has several etiologies, but often is related to obstructive hydrocephalus from meningeal cysts causing chronic arachnoiditis or from ventricular cysts producing ependymitis or directly blocking CSF pathways. Obstructive hydrocephalus should be considered a neurosurgical emergency. Occasional patients develop focal neurologic signs from a lacunar stroke due to vasculitis (32).
Headaches with or without meningismus from chronic meningitis occur in about 20% to 35% of patients. In many of these patients, the cysticercus is located in the meninges and can produce hydrocephalus (89). Children younger than 15 years of age tend to present with more seizures and less headaches than adults with neurocysticercosis (120). Recent findings suggest inflammation and free-radical release in the brain tissue surrounding the calcified lesions of neurocysticercosis, which may stimulate migraines. A case-control study was conducted, comparing migraine patients with calcified neurocysticercosis to those without. Both groups, each consisting of 78 individuals, were treated identically. At the start, patients with neurocysticercosis had a higher headache frequency, visual analog scale (VAS) score, and migraine disability assessment (MIDAS) score. After three months, this group experienced a more significant reduction in these scores than the control. This indicates that calcified neurocysticercosis lesions are not passive but rather may intensify migraine severity. Moreover, those with neurocysticercosis responded better to amitriptyline treatment, perhaps due to its anti-inflammatory properties (124).
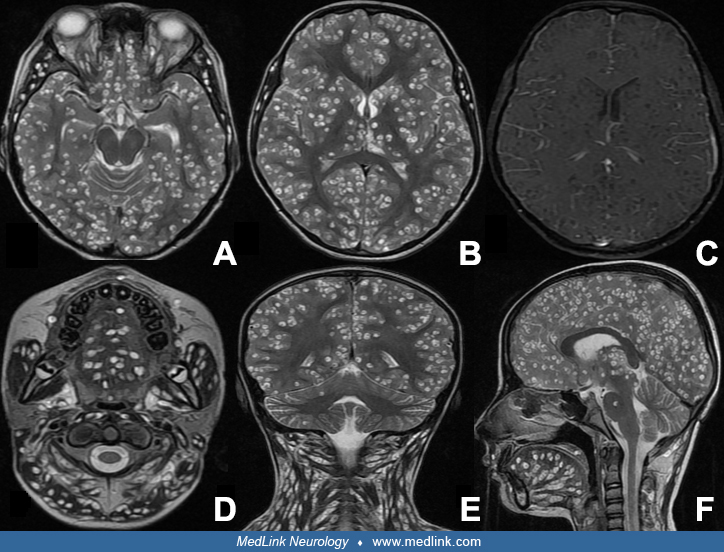
Patients with one or two cysts may never develop clinical symptoms. However, those that do tend to have seizures. The rare patient who is infested with hundreds of brain cysts will develop early clinical symptomatology, beginning weeks to months after the original infestation. Their brains may show many areas of intense inflammation with marked cerebral edema (115; 73). These patients often develop focal neurologic signs and signs of increased intracranial pressure.
Individuals with a concomitant T. solium tapeworm are at higher risk of autoinfection and have been shown to have a much higher number of brain cysticerci than individuals without a tapeworm (60). However, most individuals with CNS cysts lack gastrointestinal parasites. Individuals with only calcified cysts seldom have new neurologic signs and symptoms, but may have continued seizures.
Most patients with only parenchymal neurocysticercosis have an excellent prognosis. Many remain asymptomatic throughout the entire infection. However, those with intraparenchymal cysts often develop transient acute symptoms during cyst degeneration, but these often resolve within months to two years. Some patients develop epilepsy with either focal or generalized seizures. Usually these seizures respond well to anticonvulsant therapy. The rare patient with large numbers of CNS cysts does poorly and may die from the overwhelming CNS infection (73). Patients who develop chronic meningitis also may do poorly. Thirty percent to 60% of patients with active meningeal cysticercosis will develop obstructive hydrocephalus (44). These patients, if untreated, may experience brain herniation and death (77). The occasional patient with a cyst in the spinal cord may be left with paraparesis or quadriparesis (02). Patients who develop intraocular cysts may lose vision in the affected eye.
Neurocysticercosis is caused by CNS infection of Taenia solium larva. In neurocysticercosis, humans are the intermediate host in which cysts develop in the brain parenchyma, meninges, or ventricular spaces.
The life cycle of T. solium involves pigs and humans. Humans may be the definitive host and become infected with tapeworm or be the intermediate host and become infected with the cysticercus (90).
Man, as the definitive host with intestinal tapeworm. People become definitive hosts when they ingest insufficiently cooked pork that contains viable larvae of T. solium or larval cyst. Following ingestion of a living cysticercus, the cysticercus larva develops in the small intestine into a tapeworm 1 to 8 m in length. The tapeworm causes few clinical symptoms but can release terminal proglottids bearing up to 50,000 eggs per proglottid or 100,000 eggs per day. Terminal proglottids are passed into the stool liberating viable ova (82; 66).
If these ova are eaten by pigs through contact with contaminated human stools, the life cycle of the tapeworm continues with pigs as the intermediate hosts. The ova hatch and liberate the embryos or oncospheres that cross the intestinal wall, enter the bloodstream, and are carried into the tissues (especially muscle) where they lodge and develop into larval cysts. The mature infectious 5 to 15 mm diameter transparent cyst develops in about two months. The life cycle continues when humans eat undercooked “mealy” pork that contains the viable cysticercus.
Man, as intermediate host with neurocysticercosis. Neurocysticercosis develops when humans become the intermediate host. In about 95% of cases, this infection occurs when the individual accidentally ingests ova from a close contact with a tapeworm carrier who failed to wash his hands after defecation. Transmission from ingestion of uncooked vegetables irrigated by water contaminated with human feces occurs less often (50; 53). In the remaining 5% to 15%, the patients have an intestinal tapeworm, and the patient becomes self-infected via the fecal-oral route (86; 98; 60).
The ingested T. solium ova are partially digested in the stomach, releasing oncospheres that penetrate the stomach and intestinal mucosa to reach the bloodstream. These oncospheres may lodge in any body tissue in humans but show a predilection for the brain. Less common sites include the eye, heart, skeletal muscle, and subcutaneous tissue. In the brain, the oncospheres commonly lodge in small cerebral blood vessels located between the gray and white matter. The oncosphere then appears to burrow through the vessel wall into the adjacent brain or into the leptomeninges, often deep within the sulcus (135). Oncospheres may also lodge in the meninges, ependyma, and choroid plexus of the ventricles. Cysts involving the spinal cord are unusual (98), but have been reported to be present in patients with extraparenchymal cysticercosis (100).
Beginning weeks after ova ingestion, the larva creates a small edematous lesion in the brain. Several weeks later, the larva develops into a cyst with a protoscolex surrounded by a bladder wall. At this early stage, the cyst is tiny (1 to 3 mm in diameter). Little adjacent inflammation is present. The cyst continues to expand and becomes mature about two months after ova ingestion. The cyst has a protoscolex and a clear fluid filled bladder that is 3 to 18 mm in diameter (43). The viable cyst is often called the vesicular cyst stage. The living cyst evokes only a minimal surrounding inflammation and remains viable from 2 to more than 10 years. The mechanisms employed by helminths, including cysticercosis to prevent aggressive host inflammation against the parasite, are poorly understood. There is evidence that helminth glycans can induce an anti-inflammatory milieu via toll-like receptor 4-dependent mechanisms, which modulate host cytokine responses (140).
Over years, the osmotic barrier of the cyst wall breaks down. At this time the clear cyst fluid thickens and becomes more opaque, the cyst wall thickens, and hyaline degeneration and mineralization begin. The cyst wall begins to leak cysticercosis antigens, eliciting an intense inflammatory reaction in the adjacent brain that produces adjacent cerebral edema. The immune response is both humoral and cell mediated, including eosinophils and mast cells (119). In response to the inflammation, fibroblasts may form a capsule-like structure surrounding the cyst. The degenerating cysts are often called colloidal cyst stage. The colloidal cyst stage may persist for months to 1 to 2 years. At this stage, patients typically develop clinical symptoms. The inflammation often is sufficient to trigger seizures.
When the cysticercus dies, the bladder wall collapses to form a small granuloma. Months to years later some of these dead cysts become calcified into 1 to 6 mm nodules (called the calcified phase or nodular calcified stage); more often the lesion disappears on neuroimaging. When the cyst calcifies, some cysts develop an active perilesional gliosis, which predisposes to chronic seizures and can be detected by dynamic contrast-enhanced MRI (40; 67).
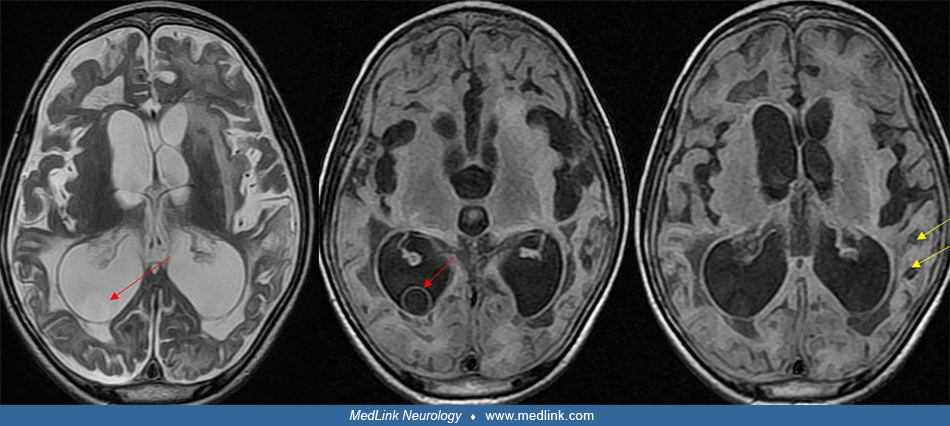
At least 5% to 15% of cysticerci lodge in the meninges or ventricular spaces. These patients usually develop clinical symptoms requiring hospitalization. It is now recognized that although less common than parenchymal cysts, extraparenchymal neurocysticercosis is presenting clinically more commonly than previously recognized (44; 123; 78). In the subarachnoid space, some cysticerci grow to over 5 cm in diameter. These giant cysticerci often produce focal neurologic signs and increased intracranial pressure with headaches and papilledema (32; 111; 05). Other cysticerci in the subarachnoid space or ventricles never develop a protoscolex and produce a grape-like or racemose structure (09; 89). These nonviable cysts frequently leak foreign antigens into the CSF, producing ventriculitis, ependymitis, chronic meningitis, or arachnoiditis. Over time, the arachnoiditis may obstruct CSF pathways, particularly at the level of the basal cisterns. Occasional lateral ventricular cysts may dislodge and travel until they reach the aqueduct of Sylvius, where they obstruct the CSF pathway, producing acute obstructive hydrocephalus (44). Patients with basal subarachnoid neurocysticercosis may also have spinal cysticercosis (14).
In a large series of neurocysticercosis, the incidence of strokes ranged from 4% to 12% (92). Most strokes occurred secondary to meningeal inflammation, causing vasculitis in adjacent blood vessels, leading to vessel thrombosis. Lacunar strokes in the basal ganglia and brainstem are the most common and have often occurred due to thrombosis of branches of the lenticulostriate artery or penetrating basilar artery branches (32).
Intramedullary cysticercosis of the spinal cord is quite uncommon. A literature review reported that most patients had a single cyst located in the thoracic spinal cord and presented as a subacute or chronic transverse myelopathy (34). The MRI demonstrates a cyst surrounded by edema with a ring-like pattern of abnormal enhancement. Some patients were treated medically but others required surgical cyst removal. Many patients made a good recovery. Meningeal cysts also can develop in the spinal meninges.
Cysticercosis is the most common parasitic infection of the human CNS. The World Health Organization, in 2010, estimated that cysticercosis has the highest global and regional disease burdens for foodborne parasitic diseases (136). Most cases of cysticercosis occur in Latin America, Africa, East Asia, India, and China (145; 100). Reports of refugees to the United States from Myanmar, Laos, Bhutan, and Burundi suggest that up to 25% have serological evidence of infection (104).
In rural Latin America, the prevalence of neurocysticercosis is quite high. One study reported a 3% prevalence, with about 6% of the inhabitants giving a history of passing tapeworm proglottids in their feces (06). Towns in rural Peru have reported neurocysticercosis prevalence rates as high as 36% (96). Two autopsy series from Brazil found a prevalence rates of 2.4% and 0.8% (85; 29). In Peru, a study reported a marked economic burden on that country’s individuals with neurocysticercosis. Patients were found to have a 54% loss of annual minimum wage salary during the first year after diagnosis, with drug costs representing 27% of the burden. Two thirds of wage earners lost their jobs, and only 61% were about to re-engage in wage-earning activities (112). On the positive side, reports from Mexico suggest the prevalence of neurocysticercosis is falling due to the better public health of its citizens (47).
According to a systematic review and meta-analysis, the proportion of neurocysticercosis among people living with epilepsy in Latin America was estimated to be 17.37% (03).
Neurocysticercosis is a growing public health problem in the United States (95). There has been an increase in the immigration of individuals from Mexico and Latin America into the United States. This immigration has resulted in an increased prevalence of neurocysticercosis in the United States, particularly in the Southwest, where it accounts for up to 10% of emergency room visits for seizures. In geographically diverse emergency rooms in the U.S., 13.5% of Hispanic patients presenting with seizures have neurocysticercosis (105). A survey of a Hispanic residential community and two farm camps in Southern California found a cysticercosis antibody prevalence of 1.8%, a frequency approaching some endemic areas in Latin America (30). Neurocysticercosis is now recognized in all states (105). The prevalence of neurocysticercosis ranges from 0.2 to 0.6 per 100,000 inhabitants in some western U.S. states (22). Neurocysticercosis rarely develops in American tourists visiting Mexico or Latin America (97) or in tourists from Israel visiting endemic countries (83). However, occasional cases are seen in U.S. citizens who have never traveled to endemic countries (98). In Houston Texas, 18% of 114 patients with neurocysticercosis were born in the United States (121; 39). A literature review of U.S. cases from 1954 to 2005 found only 78 cases, with the likely source of exposure being household members or close personal contacts in 21% (128). This may be a low estimate because tapeworms usually persist in the intestine only a few years. However, their contacts consuming the ova may not develop degenerating cysts for many years, so searching for ova in the stools of household members may not yield the source (64).
The cost of hospitalization for neurocysticercosis is considerable. United States data from 2003 to 2012 estimated 23,266 hospitalizations with a mean hospital stay of 6 days and a cost of $1,149,000 (103). Seizures, obstructive hydrocephalus, and headaches were the most common reasons for hospitalization. An analysis of treatment costs in Mexico City, Mexico reported the average direct costs were U.S. $503 for outpatient care and U.S. $2506 when hospitalization was necessary (07).
Patients with neurocysticercosis in the endemic country can be of any age, from early childhood to elderly, but the majority of cases are in older children and young adults. Most cases in the U.S. are discovered in those immigrating to the U.S. and are young adults, with a mean age of 27 years (123; 23).
To prevent development of the intestinal tapeworm, all pork should be thoroughly cooked prior to eating (08). Freezing pork to -20°C for several days will also inactivate cysticerci (131). Smoked or dried pork may still contain viable cysticerci. Prevention of neurocysticercosis is accomplished by avoiding ova-contaminated food and water. In endemic areas, consumption of raw vegetables should be avoided because they may be contaminated with human fertilizer. Heating food above 60°C or freezing below -30°C is usually sufficient to kill ova (82). Restaurant workers from endemic areas should have their stools routinely checked for the presence of tapeworm ova. However, American tourists visiting Mexican and South American countries appear to be at little risk of developing either the tapeworm or neurocysticercosis unless they visit rural areas.
Progress is being made in the development of a vaccine to prevent cysticercosis in pigs; the vaccine could prevent transmission of the pig tape worm to humans if widely used in Latin American countries (84). However, the vaccine cost to a poor farmer will be a problem for widespread usage.
The differential diagnosis of neurocysticercosis depends on the type of clinical presentation. If cysts are identified on CT or MRI scan, major diagnoses to be considered include tuberculoma, brain abscess, syphilitic gumma, arteriovenous malformation, metastatic tumor, small primary tumor, or other parasitic cysts, such as schistosomes or amoebas. Tuberculomas tend to be larger than 20 mm in diameter, have an irregular outline, cause more mass effect, and tend to produce a progressive focal neurologic deficit, whereas T. solium cysts tend to be less than 20 mm in diameter, have smooth regular outline, and seldom cause progressive focal neurologic deficits (131). Diffusion-weighted imaging typically shows hyperintense brain abscesses with low apparent diffusion coefficient values compared to non-brain abscess lesions such as neurocysticercosis (116).
At times, neuroimaging of cysticercosis resembles malignancy, but it can often be distinguished using 18F-FDG PET/MRI (75). A review on ocular parasitic disease nicely discusses the differential diagnosis of ocular parasites (25).
If the patient presents with a subacute or chronic meningitis or obstructive hydrocephalus, tuberculous meningitis, fungal meningitis, cerebrovascular syphilis, neurosarcoidosis, meningeal carcinomatosis, and CNS vasculitis need to be considered. The presence of CSF eosinophils above 10% increases the probability of meningeal cysticercosis but is uncommonly present.
The diagnosis of neurocysticercosis should be considered, especially in young adults from countries endemic for cysticercosis who present with the new onset of focal or generalized seizures, unexplained subacute meningitis, obstructive hydrocephalus, unexplained strokes, or CNS cystic masses. The clinical diagnosis is established if the CT or MRI demonstrates intraparenchymal cysts showing the protoscolex, or a typical cystic lesion in a patient who has anticysticercus antibodies in CSF or serum.
Neuroimaging with CT or MRI is extremely helpful (28). The MRI is more sensitive than CT for detection of cysts, and the delayed gadolinium-enhanced T1 sequence is the most sensitive (87). There are two excellent reviews of neuroimaging in the stages of neurocysticercosis (70; 139). MRI sequences called Fast Imaging Employing Steady-sTate Acquisition (FIESTA), T2 Star-Weighted ANgiography (SWAN), and SPoiled Gradient Recalled echo (SPGR) were able to detect the cyst scolex in 83% compared to 45% by routine sequences (81). This is a huge advantage in distinguishing tuberculomas from neurocysticercosis. CT is more sensitive than MRI for detecting calcified inactive cysts.
Early in the infection, CT scans usually show small homogeneous contrast enhancing lesions that are somewhat ill defined. As the cyst matures, 3 to 18 mm noncontrasting enhancing cysts will be seen without significant adjacent edema. These cysts are typically scattered throughout the brain parenchyma often near the gray-white matter junctions. As the cyst begins to degenerate, neuroimaging demonstrates a ring-contrast-enhancing cyst with adjacent edema. MRI scan with the administration of gadolinium demonstrates ring enhancement on T1-weighted images.
Occasionally, giant cysts are seen measuring several centimeters in diameter. After the cyst has degenerated, the cyst walls collapse and the cyst becomes isodense to brain parenchyma on CT scan. Over months to years, some cysts will calcify and be identified as calcific nodules 2 to 6 mm in diameter. These calcific nodules are often difficult to identify by MRI (149). Contrast enhancement and perilesional edema and gliosis may be seen around old calcifications in association with symptoms such as seizures (101; 149; 102; 110). Some patients may have both living cysts and calcified nodules due to repeat infections or delayed deaths of some cysts. In patients from endemic areas who often become repeated-infected, it is common to see patients whose CT scan shows both living cysts and calcified nodules. In developing countries, distinction between a tuberculoma and cyst from neurocysticercosis can be challenging. Identification of a protoscolex within the cyst diagnoses cysticercosis. In its absence, diffusion weighted MRI typically shows higher apparent diffusion coefficients in cysticercosis than tuberculomas (68). Advanced MRI sequences such as diffuse-weighted imaging (DWI/DTI), susceptibility-weighted imaging (SWI), 3-dimensional constructive interference in steady state (CISS), perfusion MR imaging, and magnetic resonance spectroscopy (MRS) can be helpful in diagnosing difficult brain cysts (139).
Identification of cysts within the ventricles or meninges is often difficult and may have a varied presentation (117; 44). The cyst fluid is usually isodense with CSF and may not enhance with contrast on CT. A study reported that use of intrathecal gadodiamide improves identification of subarachnoid and ventricular cysts even when cysts are not shown by conventional neuroimaging (71). The cyst may be detected on the basis of distortion or disproportionate enlargement of the third or fourth ventricles. Use of nonionic contrast CT ventriculography may outline the cyst (148; 132). However, in general the cysts are best detected by MRI. T2-weighted MRI images often do not detect viable intraventricular cysts but they can be distinguished from CSF on proton density images (61; 44). T1-weighted images often demonstrate a thin bladder wall with a protoscolex within the CSF-isointense cystic fluid.
Demonstration of cysticercosis lesion outside the central nervous system helps in the confirming diagnosis neurocysticercosis. Histopathologic demonstration of cysticercus larva in biopsied specimen of subcutaneous nodule is virtually confirmatory. Calcified lesions (cigar-shaped lesions) in muscles are seen occasionally.
Concomitant HIV infection complicate the diagnosis and treatment of patients with neurocysticercosis. Immunocompromised patients with a low CD4 count present with atypical lesions, resulting in delayed diagnosis. Differential diagnosis with similar looking lesions of toxoplasmosis becomes difficult. Early treatment with highly active antiretroviral therapy results in patients presenting with more classical symptoms and imaging appearances (80).
Results of blood hemograms, including the number of eosinophils, are usually normal. Table 2 outlines the common CSF findings in patients with neurocysticercosis. A CSF pleocytosis can result from a colloid intraparenchymal cyst located near the meninges leaking mononuclear cells into the CSF or from meningeal cysticercosis.
|
CSF finding |
Percentage |
|
• Elevated opening pressure (> 200 mm H20) |
25% |
|
|
Serologic tests for cysticercosis have improved. As of 2022, the most sensitive and specific diagnostic test is an enzyme-linked immunoelectrotransfer blot assay that was initially developed by the Centers for Disease Control and Prevention (137). Studies have shown serum testing is highly sensitive (95%) and specific (98%) for patients with multiple cysts but less sensitive in detecting solitary cysts (28% to 70%) (147; 55; 59). After the cysticercus dies, the patient’s cysticercus antibody titer persists for a long time but eventually falls to undetectable levels. Thus, patients with only calcified cysts have a positive test ranging up to 88% for patients with multiple calcified cysts to as low as 10% for solitary calcified cysts. In general, the serum enzyme linked immunoelectrotransfer blot assay is as sensitive and specific as the test done on CSF (118).
Older serologic tests use unfractionated cysticercus antigens and include the enzyme-linked immunosorbent assay and complement fixation assay. These tests are cheaper but are associated with high rates of false positive and false negative reactions, giving the tests a sensitivity and specificity of about 70%. The sensitivity and specificity of these tests improves when CSF rather than serum is assayed.
An IgG monoclonal antibody-based antigen detection enzyme-linked immunosorbent assay (Ag-ELISA) has been developed. The test is sensitive (up to 86%) for detection of cysticercus antigen in CSF, especially in meningeal cysticercosis. The antigen test may be helpful to diagnose atypical cysticercosis lesions and could be used to follow response to albendazole treatment (11; 118; 45). The test is less sensitive in serum, especially when only calcified lesions are present. A polymerase chain reaction assay to detect T. solium DNA in CSF has shown a sensitivity of 67% if unconcentrated CSF is used but a sensitivity of 96.7% when the CSF is concentrated. This test is becoming more available, and it can also be used to follow clearance of the parasite in patients with meningeal cysticercosis. A modification of the antigen detection assay has been successfully used on urine in developing countries where rural patients were reluctant to have their blood or CSF drawn (19). However, the urine assays are less sensitive than serum or CSF assays.
Coming in the near future are global DNA screening platforms to detect specific DNA sequences from many infectious organisms simultaneously, using ribosomal RNA (rRNA) genes sequencing. A case report using this technology identified two biopsied space occupying brain masses as due to cysticercosis when standard histology of the tissue could not (69). Whether global DNA screening platforms are sensitive enough to detect cysticercosis in CSF in patients with chronic meningitis is currently unclear.
The utilization of next-generation sequencing on cerebrospinal fluid presents a promising approach for the accurate diagnosis and continuous monitoring of neurocysticercosis. A 68-year-old Brazilian woman exhibited fatigue, walking difficulties, and weight loss. Examination revealed gait apraxia and executive dysfunction. Brain MR indicated hydrocephalus, and cerebrospinal fluid abnormalities pointed to an infection. Metagenomic next-generation sequencing diagnosed neurocysticercosis (109; 144).
Table 3 compares the relative sensitivity of serological neurocysticercosis tests performed on CSF from patients with proven neurocysticercosis.
|
Cyst location by neuroimaging |
Antibody test# |
Antigen test+ |
PCR assayα |
|
| |||
In 2017, international cysticercosis experts published a revised diagnostic criterion for neurocysticercosis (see Table 4) (36). The criteria updated current diagnostic criteria and emphasized neuroimaging studies as an important criterion.
|
Absolute criteria: | |
|
• Histologic demonstration of parasite from biopsy of brain or spinal cord lesion. | |
|
• Visualization of subretinal cysticercus. | |
|
• Conclusive demonstration of a scolex within a cystic lesion on neuroimaging. | |
|
Neuroimaging criteria: | |
|
Major neuroimaging criteria: | |
|
• Cystic lesions without a discernible scolex. | |
|
• Enhancing lesions. | |
|
• Multilobulated cystic lesions in the subarachnoid space. | |
|
Confirmative neuroimaging criteria: | |
|
• Resolution of cystic lesions after cysticidal drug therapy. | |
|
• Spontaneous resolution of single small enhancing lesions. | |
|
• Migration of ventricular cysts documented on sequential neuroimaging studies. | |
|
Minor neuroimaging criteria: | |
|
• Obstructive hydrocephalus (symmetric or asymmetric) or abnormal enhancement of basal leptomeninges. | |
|
Clinical/exposure criteria: | |
|
Major clinical exposure: | |
|
• Detection of specific anticysticercal antibodies or cysticercal antigens by well-standardized immunodiagnostic tests. | |
|
• Cysticercosis outside the central nervous system. | |
|
• Evidence of a household contact with T. solium infection. | |
|
Minor clinical exposure: | |
|
• Clinical manifestations suggestive of neurocysticercosis. | |
|
• Individuals coming from or living in an area where cysticercosis is endemic. | |
|
Degrees of diagnostic certainty | |
|
Definitive diagnosis: | |
|
• One absolute criterion. | |
|
• Two major neuroimaging criteria plus any clinical or exposure criteria. | |
|
• One major and one confirmative neuroimaging criteria plus any clinical or exposure criteria. | |
|
• One major neuroimaging criteria plus two clinical or exposure criteria (including at least one major clinical/exposure criterion) together with the exclusion of other pathologies producing similar neuroimaging findings. | |
|
Probable diagnosis: | |
|
• One major neuroimaging criteria plus any two clinical or exposure criteria. | |
|
• One minor neuroimaging criteria plus at least one major clinical or exposure criteria. | |
|
| |
The management of neurocysticercosis is usually medical with antihelminthic drugs, corticosteroids, and anticonvulsants. The main indications for surgery include cysts exhibiting local compression of brain or cranial nerves, hydrocephalus, intraventricular cysticercosis, spinal cysticercosis with root or cord compression, and ocular cysts (127). Optimal management depends on the number, location, and viability of the cysts. Table 5 lists the current treatment recommendations based on the literature (127; 114; 52; 04; 31; 57) as modified by my personal experience in treating patients in the United States.
|
Type of neurocysticercosis |
Recommendations* | ||
|
Parenchymal cysts | |||
|
• Viable vesicular cysts | |||
|
- One to three cysts on neuroimaging |
Antihelminthic drugs ± steroids | ||
|
• Degenerating colloid cysts | |||
|
- One to three cysts on neuroimaging |
Antihelminthic drugs ± steroids | ||
|
• Dead calcified cysts (asymptomatic) |
No antihelminthic treatment | ||
|
Cysticerotic encephalitis |
High dose steroids without antihelminthic drugs | ||
|
Giant cysts |
Albendazole with steroids; CSF shunting if hydrocephalus develops | ||
|
Meningeal cysts | |||
|
• With hydrocephalus |
CSF shunting, albendazole with steroids often in high dosage repeatedly for years, careful monitoring of patient for shunt malfunction | ||
|
• Without hydrocephalus |
Albendazole in one to two courses plus steroids with careful monitoring of patient for possible hydrocephalus | ||
|
Intraventricular cysts | |||
|
• With hydrocephalus |
CSF shunting, careful monitoring of patient for shunt malfunction, albendazole with steroids; surgical removal of cyst when possible, especially via endoscope | ||
|
• Without hydrocephalus |
Surgical removal of cyst when possible, especially via endoscope; plus albendazole and steroids | ||
|
Spinal cysts |
Surgical cyst removal with steroids, or albendazole with steroids, when possible | ||
|
Ophthalmic cysts | |||
|
• Cyst in globe |
Surgical cyst removal | ||
|
• Cyst outside globe |
Antihelminthic drug with steroids or surgical cyst removal, when possible | ||
|
| |||
Albendazole and praziquantel are FDA-approved for treatment of neurocysticercosis. Albendazole is moderately well-absorbed by oral administration especially when given with a fatty meal. The drug is rapidly metabolized in first passage by the liver to its active sulfoxide metabolite (130), of which 70% is bound to protein. The sulfoxide plasma half-life is between 8 and 14 hours, and it is eliminated in urine with an elimination half-life of 8.5 hours (130). The drug readily crosses the blood-brain and blood-CSF barriers to achieve concentrations in CSF that are higher than corresponding serum concentrations and higher than that achieved by praziquantel. However, the sulfoxide concentration in cyst fluid is lower than that of plasma levels but is sufficient to kill the cyst. Albendazole is well-tolerated with minimal adverse effects that include dizziness, gastrointestinal distress, rashes, leukopenia, and elevated serum liver enzymes. Albendazole has been shown to cause teratogenic effects in rats and rabbits and should be avoided in pregnancy. The mechanism by which the cyst dies is incompletely understood but the drug seems to cause degeneration of parasite cytoplasmic microtubules impairing glucose intake, which leads to energy depletion and parasite starvation (31). The dose of albendazole is usually 15 mg/kg per day divided into two doses for 8 to 14 days (130; 31), but other regiments have been published. For extraparenchymal neurocysticercosis, treatment with 30 mg/kg/day was reported to be superior to 15 mg/kg/day (63). Concomitant administration of corticosteroids has been shown to increase serum albendazole concentration by 50% (106). Administration of anticonvulsants or other medications does not affect the serum drug concentration.
Praziquantel is well absorbed orally and undergoes extensive first passage hepatic metabolism with the metabolites being inactive (130). Peak plasma levels occur 1.5 to 2 hours after administration. About 80% of praziquantel is bound to plasma, but free praziquantel rapidly is distributed in body tissue due to its high lipid solubility. Praziquantel traverses the blood-brain and blood-CSF barriers to achieve therapeutic concentrations in CSF and within cyst fluid (106; 130). Praziquantel has a plasma and elimination half-life of 1.5 to 2.5 hours. The drug is well-tolerated with few side effects that include gastrointestinal distress, dizziness, fever, headache, and occasionally a diminished sense of well-being (79). Praziquantel has not been shown to be teratogenic in animals. The mechanism of action of praziquantel is poorly understood but the drug appears to affect calcium channels on the surface of the parasite, causing muscle contractions producing spastic paralysis of the parasite (138; 127; 31). An in vitro assay reported that exposure to praziquantel caused reduced cyst size and inhibition of evagination (91). However, the drug may not kill the racemose form of neurocysticercosis, which lacks a protoscolex. The usual dose of praziquantel is 50 mg/kg per day divided into three doses for 15 days (129). Unlike albendazole, concomitant administration of corticosteroids, phenytoin, or carbamazepine will decrease serum and CSF levels of praziquantel (129; 10). Concomitant administration of praziquantel with cimetidine or a high fat or high carbohydrate diet elevates serum praziquantel levels (20).
Although both albendazole and praziquantel are effective and equally safe against the cerebral parasite, two meta-analyses of comparative trials reported that albendazole is slightly more effective in reducing the cyst burden and controlling seizures (37; 93). Garcia and colleagues have reported that for patients with many cysts, a combination of both albendazole and praziquantel together was superior in eliminating the cysts at 6 months than either alone without producing any increase in side effects (51; 56). However, an older reference did not find benefit in children treated with both drugs (76).
Corticosteroid administration along with the antihelminthic drug is often indicated to minimize transient worsening of clinical symptoms that may occur early in the treatment. Both antihelminthic drugs rapidly kill the viable cyst releasing antigenic material into the surrounding brain or CSF. As a consequence, there can be a marked increase in inflammation and cerebral edema that can produce new or increased clinical signs that include: increased intracranial pressure (with headaches, lethargy, nausea and vomiting), seizures, and focal neurologic signs such as hemiparesis, ataxia or visual loss. The symptoms often begin on day 2 to 3 of treatment and last 3 to 5 days (130). When albendazole is used, the corticosteroids should be given simultaneously and continued until day 5 or longer if there is a large parasite burden or a severe reaction develops. Dexamethasone, 8 to 24 mg per day in four divided doses orally, intramuscularly, or intravenously or prednisone 1 mg/kg per day orally are usually given (130; 31). When praziquantel is administered, the corticosteroids are often withheld for 1 or 2 days or longer if possible because steroids decrease serum praziquantel levels by 50% (20). If increased symptoms develop, steroids are then administered intramuscularly or intravenously for rapid effect and continued as long as necessary.
Although there is some controversy as to whether treatment with antihelminthic drugs improves resolution of seizures in patients having only one or two intraparenchymal cysts, a 2013 guideline review found that most studies favored albendazole treatment. A 2010 Cochrane collaboration reported that for adults, no difference was detected for albendazole compared with no treatment for recurrence of seizures, but fewer participants with albendazole had lesions at follow up (114). The argument for antihelminthic treatment is based on shortening the time to cyst disappearance on CT and MRI and killing all cysts simultaneously, preventing prolongation of brain inflammation due to multiple cysts degenerating at different times (27). A double-blind, placebo-controlled study of 120 Peruvian patients with seizures and neurocysticercosis lesions identified by neuroimaging reported a significant 67% reduction in seizures with generalization following treatment with albendazole. There was also a trend for reduction of partial seizures (58). A meta-analysis also concluded that cysticidal drug therapy results in better resolution of colloidal and vesicular cysticerci, lower risk of recurrence of seizures in patients with colloidal cysticerci, and a reduction in the rate of generalized seizures in patients with vesicular cysticerci (37). In the United States, most patients with cysticercosis receive antihelminthic treatment.
Anticonvulsants should be administered to all patients with seizures. Anticonvulsants should be continued for 1 or 2 years or until the active cysts have disappeared on neuroimaging and the brain irritation has subsided (18). Commonly prescribed anticonvulsants are phenytoin, valproate, and carbamazepine (52). About 80% of patients do not subsequently develop seizures after the anticonvulsants are discontinued. Patients with subsequent seizures should be considered to have epilepsy from unprovoked seizures and restarted on long-term anticonvulsants (18). One study reported a higher rate of long-term seizures in patients whose cysts calcified rather than just degenerated (62). The long-term clinical outcome of parenchymal neurocysticercosis from India in 500 children found that single lesions have a favorable outcome with few seizure recurrences (126).
The optimal duration of antihelminthic treatment for extraparenchymal neurocysticercosis is unknown, but probably of longer duration than for parenchymal disease (49). Despite apparent disappearance of viable parasites, chronic inflammation around undetected parasitic membranes may ensue with disease continuation or progression. A specific antigen detection ELISA assay that detects cysticercosis antigens has been developed (54; 46). Monitoring CSF or serum antigen levels may help evaluate efficacy and duration of antiparasitic and steroid therapy. There is some evidence that detection of cysticercus antigen in CSF may help diagnose meningeal extraparenchymal neurocysticercosis in patients with chronic meningitis (108).
Intraventricular neurocysticercosis. Intraventricular vesicular cysts often can be removed surgically, often by endoscopic surgery (72; 113). However, if the cyst is degenerating and producing a ventriculitis, the cyst is often firmly attached to ependyma or choroid plexus and difficult to remove surgically. Small viable cysts in the lateral ventricle have successfully been treated with albendazole. Because both drugs act by killing the cysticercus, the drugs are not useful in treatment of patients with dead calcified cysts. Guidelines recommend removal of the cyst by minimally invasive neuroendoscopy over other surgical or medical methods. Antihelminthic drugs with corticosteroid therapy after shunt insertion is recommended to decrease subsequent shunt failure in patients in whom surgical removal of isolated intraventricular cysts is not possible (146; 125).
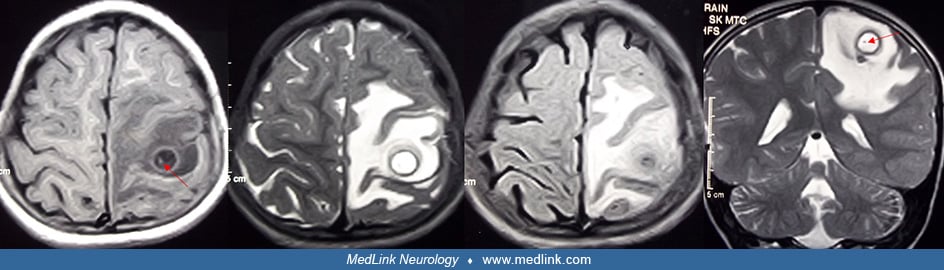
Solitary cysticercus granuloma. Solitary cysticercus granuloma is the most frequent type of neurocysticercosis in many countries, such as India. Solitary cysticercus granuloma often presents with new-onset epilepsy. On neuroimaging, solitary cysticercus granuloma appears as an enhancing ring-shaped lesion. These lesions are usually less than 20 mm in diameter and are surrounded by perilesional vasogenic edema. In many lesions, eccentric dot representing scolex is seen. Immunodiagnosis is often not helpful in the patients with single cysticercal lesions. Patients with solitary cysticercus granuloma require antiepileptic treatment. The role of albendazole and corticosteroids is controversial. Prognosis is generally good as the majority resolves spontaneously. In some patients, lesions get calcified. Antiepileptic therapy can safely be withdrawn after the lesion has disappeared. Calcification and perilesional gliosis are responsible for seizure recurrences in some cases (48; 88).
Infrequently, solitary cysticercus granuloma are seen in the brainstem. Such patients present with a variety of intrinsic brainstem syndromes. These lesions require treatment with corticosteroids only (33).
Suthar and colleagues, in a prospective follow up study, noted that in approximately one third of patients with solitary cysticercus granuloma, the neurocysticercosis lesions get calcified (133). Diffusion restriction within the lesion, a calcified nodule within the lesion, and lesion size greater than 10 mm at baseline predicted calcific transformation of the cysticercus granuloma during follow-up.
Calcified neurocysticercosis. Cysticercus granuloma usually resolves leaving a calcified nodule. Most calcified neurocysticercosis lesions are asymptomatic, but many cases with calcified neurocysticercosis lesions are associated with recurrent seizures. Development of perilesional edema because of inflammatory changes in brain parenchyma at lesion site is considered the cause for seizures. Calcified neurocysticercosis lesions if associated with seizures require use of antiepileptic drugs. Albendazole is not needed as calcified lesion represents a dead parasite (99).
A meta-analysis noted that approximately 38% of parenchymal neurocysticercosis cysts get calcified following antiparasitic treatment. Predictors for cyst calcification were cysts larger than 14 mm, cysts with perilesional edema, seizures persisting for more than 24 months, mild antibody response, high albendazole regime, lower doses of corticosteroids, and no early antiparasitic re-treatment (13).
In patients with calcified neurocysticercosis, intermittent perilesional edema may develop, often coinciding with a seizure recurrence. A patient with calcified neurocysticercosis demonstrated, on repeated MR imaging, that during a follow-up of four years, the seizure recurrences were associated with recurrent perilesion edema and contrast enhancement. Perilesional edema disappeared in the seizure-free periods. It was also noted that perilesional edema each time occurred around only one particular calcified nodule. Location of calcified lesion coincided with the EEG findings and seizure semiology. The patient, in fact, had many more brain calcifications (74).
Subarachnoidal neurocysticercosis. The subarachnoidal form of neurocysticercosis is aggressive. Subarachnoidal neurocysticercosis frequently causes intracranial hypertension due to obstruction in cerebrospinal fluid flow leading to development of hydrocephalus. Subarachnoidal neurocysticercosis also leads to an inflammatory basal arachnoiditis involving deep penetrating vessels. Subacute to chronic hydrocephalus requires cerebrospinal fluid shunt surgery. 2017 Clinical Practice Guidelines recommend that these patients be treated with antihelminthic therapy (146). Death may occur from shunt malfunction or from vasculitis of brainstem blood vessels resulting in brainstem or thalamic infarction (32).
Disseminated neurocysticercosis. So far, management of disseminated cysticercosis is unclear and treatment with albendazole largely considered hazardous. In a prospective evaluation, patients with disseminated cysticercosis, after excluding cysticercosis encephalitis and subretinal cysticercosis, were treated with three cycles of 4-week albendazole. Follow-ups were done three months after a treatment cycle. After third follow-up, there were significant reductions in lesion load. There was a significant reduction in the occurrence of seizures and headache as well. No patient developed serious side-effects (107). In a case series, Agarwal and co-workers treated three patients with heavy neurocysticercosis load with albendazole and noted significant reduction in lesion load following treatment (01). None of the three patients had any major adverse reaction following treatment. Del Brutto and Garcia suggested that patients with heavy nonencephalitic cysticercosis should be treated with a simpler treatment regimen consisting of lower doses of a single drug instead of a combination of two antiparasitic drugs (35). Corticosteroids should be started before the start of antiparasitic treatment and may be continued several days after antiparasitic treatment is completed. Wangda and colleagues investigated the effects of albendazole on disseminated neurocysticercosis, a condition with widespread cystic lesions in the brain and other body parts (142). Of 35 patients screened with multiple brain lesions, 13 had disseminated neurocysticercosis. These patients underwent whole-body MRI scans, revealing an average of 163.6 brain lesions and 629.9 lesions elsewhere in the body. After albendazole treatment, brain lesions reduced significantly to an average of 99, and extracerebral lesions decreased to an average of 183.4. The study concludes that whole-body MRI is crucial for patients with multiple brain neurocysticercosis and that albendazole effectively reduces lesion counts (142).
Cysticercosis encephalitis. Cysticercosis encephalitis is a life-threatening form of neurocysticercosis associated with a massive infestation of the brain. Cysticercosis encephalitis is characterized by marked brain edema and signs of raised intracranial tension. Albendazole therapy may prove disastrous for such patients. Ideally, these patients are treated symptomatically and with corticosteroids (73).
Eliminating neurocysticercosis from developing countries remains a challenge, especially when rural individuals raise pigs on their property. However, pilot studies using a highly effective pig cysticercosis vaccine have shown that interruption of pig to human transmission is feasible (57). Unfortunately, the cost of widespread use of the pig vaccine is currently too high to use widely. Albendazole, a pregnancy category C drug, has been shown to be teratogenic in animal models.
Neurocysticercosis as a cause of seizures should be considered in pregnant or postpartum women, particularly if coming from endemic areas. Its diagnosis and management during pregnancy can be challenging, but prompt diagnosis and treatment are crucial for better maternal and fetal outcomes (143). Cunningham and Twickler shared their experience of 37 pregnant women with neurocysticercosis (24). Five pregnant women were asymptomatic. Among 32 symptomatic women, severe headaches and new-onset seizures were the most frequent clinical manifestations. Neurocysticercosis was diagnosed after workup for a history of neurocysticercosis or evaluation of a pre-existing seizure disorder. All of these patients were medically treated with albendazole, praziquantel, or both, along with corticosteroids or antiepileptic drugs, or both. In eight patients, neurosurgical procedures such as shunt surgery were performed. The majority of patients (32 out of 37) delivered full-term healthy babies. In one case there was premature delivery at 34 weeks. In four, pregnancy was lost, representing two molar pregnancies, one miscarriage, and one stillbirth.
Neurocysticercosis normally is not affected by administration of anesthesia. However, patients with increased intracranial pressure from the neurocysticercosis may be more sensitive to anesthesia. Anesthetic drugs that do not increase intracranial pressure should be preferentially used.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Ravindra Kumar Garg DM FRCP
Dr. Garg of King George's Medical University in Lucknow, India, has no relevant financial relationships to disclose.
See Profile
John E Greenlee MD
Dr. Greenlee of the University of Utah School of Medicine has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Infectious Disorders
Oct. 08, 2024
Infectious Disorders
Sep. 13, 2024
Infectious Disorders
Aug. 27, 2024
Infectious Disorders
Aug. 27, 2024
Infectious Disorders
Aug. 26, 2024
Infectious Disorders
Aug. 05, 2024
Infectious Disorders
Jul. 11, 2024
Infectious Disorders
Jul. 03, 2024