Infectious Disorders
Zika virus: neurologic complications
Oct. 08, 2024
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Poliovirus is an acute enteroviral infection that is spread from person to person, primarily via the fecal-hand-oral routes. Although most patients who acquire the infection are asymptomatic, those afflicted may develop a variety of neurologic manifestations, including aseptic meningitis, polioencephalitis, bulbar poliomyelitis, and paralytic poliomyelitis. In paralytic poliomyelitis, muscle weakness is preceded by intense myalgias of the involved limbs and axial skeleton. Following recovery, as many as 20% to 30% of individuals who develop paralytic poliomyelitis may suffer from post-polio syndrome, which produces muscle weakness, pain, atrophy, and fatigue many years after acute illness.
There has been a large worldwide effort for poliomyelitis eradication. Polio cases have decreased by over 99% since 1988, from an estimated 350,000 cases in more than 125 endemic countries to 42 reported cases in 2016 (37 wild-type and 5 vaccine-derived) (74; 81; 156; 157; 169; 84; 102). Poliomyelitis due to wild-type virus has now been eliminated from the Americas, Europe, and Western Pacific, and cases in Africa and Asia have markedly decreased. Although great strides have been made, poliomyelitis remains and is rising in some regions, including Nigeria, Afghanistan, and Pakistan, and there is a risk for new outbreaks to occur.
|
• Poliovirus is an acute enteroviral infection that is spread from person to person, primarily via the fecal-hand-oral route. | |
|
• Neurologic manifestations of poliomyelitis include aseptic meningitis, polioencephalitis, bulbar poliomyelitis, and paralytic poliomyelitis. | |
|
• As many as 20% to 30% of individuals who develop paralytic poliomyelitis may suffer from post-polio syndrome, which includes new muscle weakness, pain, atrophy, and fatigue many years after the acute illness. | |
|
• There has been a large worldwide effort for poliomyelitis eradication, with a decrease of polio cases by over 99% since 1988. Though great strides have been made, there remains a high risk for new outbreaks. | |
|
• On September 20, 2015, the Global Commission for the Certification of Poliomyelitis Eradication declared that poliovirus type 2 has been eradicated worldwide. |
Poliomyelitis has afflicted humans for centuries. The first recognized clinical description was by English physician and surgeon Michael Underwood (1736-1820) in the second edition of A Treatise on Diseases of Children, which was published in 1789 (235; 141; 186). Major contributions to the understanding of the disease were made by the German orthopedist Jacob Heine (1800-1879), who described the clinical features of acute poliomyelitis (96). In 1870, French neurologist Jean-Martin Charcot (1825-1893) and his junior colleague Alix Joffroy (1844-1908) recognized that the flaccid paralysis of poliomyelitis was caused by spinal anterior horn cell damage (49).
In the mid- to late 19th century, the general belief was that polio was not infectious; in the late 19th century, when bacteriological explanations were in vogue for almost all conditions, polio was suspected to be another bacterial disease. Numerous attempts were made to identify the responsible microorganism, with many claims to have successfully done so, but none were reproducible.
Finally, in 1908, Austrian pathologist Karl Landsteiner (1868-1943) and his colleague Erwin Popper (1879-1955) showed that the etiological agent of poliomyelitis was a filterable virus (127). The existence of more than one type of poliovirus was first inferred by Australian virologist Frank Macfarlane Burnet (1899-1984) and his collaborator Dame Annie Jean Macnamara (1899-1968) in 1931 when they demonstrated that monkeys who had recovered from infection with a strain recovered in Melbourne subsequently developed disease when given a virulent mixed virus strain (40; 70). In 1949, the three antigenic poliovirus types (poliovirus 1, poliovirus 2, poliovirus 3) were identified (34; 119).
The first recorded outbreaks of poliomyelitis occurred in the mid- and late nineteenth century in northern Europe and North America, followed by much larger epidemics in the early 20th century.
In August 1921, while vacationing with his family at their summer home on Campobello Island off the coast of Maine, politician Franklin Delano Roosevelt (FDR; 1882-1945) became ill, lost motor power in his legs, and was diagnosed with polio. Roosevelt later sought treatment at a resort in Warm Springs, Georgia. Because of his self-perceived improvement at the resort, in 1927, Roosevelt and his friend, American lawyer Basil O’Connor, created the Georgia Warm Springs Foundation, in which O'Connor served first as treasurer and later as president. The foundation was subsequently reconstituted as the National Foundation for Infantile Paralysis in 1938. A solicitation prior to Roosevelt’s birthday in 1938 resulted in a huge influx of small donations that swamped the White House mail room. As a result, radio star Eddie Cantor dubbed it the “March of Dimes” -- a play on the contemporary radio and newsreel series, The March of Time. The March of Dimes ultimately became the official name of the organization, which served as the largest source of funding for research and clinical care for poliomyelitis at the time. Indeed, the organization supported the work of Jonas Salk and others, which led to the development and testing of polio vaccines.
Prototype “iron lung” negative-pressure ventilators were developed in the late 1920s and early 1930s by industrial hygienist Philip A Drinker (1894-1972), physiology instructor Louis Agassiz Shaw Jr. (1886-1940), and inventor/medical-equipment manufacturer John Haven (“Jack”) Emerson (1908-1997). From around 1926 to 1928, Drinker and Shaw, both at Harvard Medical School in Boston, designed an electrically powered tank respirator. On October 13, 1928, Drinker and pediatrician Charles F McKhann demonstrated the potential of this device in an 8-year-old girl with poliomyelitis, respiratory failure, and coma who was treated at Boston Children’s Hospital and briefly survived before succumbing to pneumonia. A second trial on Friday, September 13, 1929, at Peter Bent Brigham Hospital in Boston, on a 21-year-old Harvard student, was unquestionably successful: Hoyt was weaned from the respirator in 4 weeks and was discharged from the hospital before Christmas. Emerson built a mechanically superior device in the summer of 1931; Emerson’s device was first used clinically to save the life of a priest with polio at the Providence City Hospital in Providence, Rhode Island.
After this, the manufacture of "iron lungs" expanded markedly over the next 2 decades. At their peak use in the early 1950s, wards at some referral centers were crowded with dozens of these devices, all in use for affected patients, with a large complement of attendant nursing and respiratory therapy staff. Indeed, the iron lung required intensive nursing care and respiratory therapy and a supporting hospital infrastructure.
An Emerson Respirator, also known as an iron lung, was a machine that enabled a person to breathe on their own when muscle control had been lost (often due to poliomyelitis). It used negative pressure ventilation to induce inha...
(Creator: U.S. Air Force. File name: 244001_1370. Citation: Mayor John F Collins records, Collection #0244.001, City of Boston Archives, Boston. Creative Commons Attribution 2.0 Generic License [CC BY 2.0], https://creativecomm...
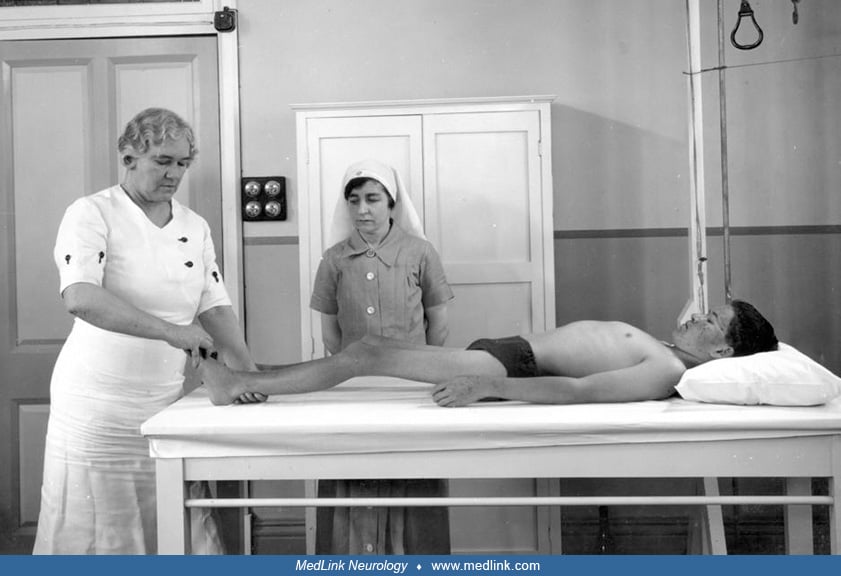
From the 1920s through at least the early 1940s, the orthodox treatment for poliomyelitis consisted largely of absolute and prolonged immobilization of affected limbs with splints or plaster casts, followed by often permanent orthopedic braces; in retrospect, this approach was less than ideal, and in many ways counterproductive as it caused or contributed to disuse atrophy, joint contractures, and lifelong disability. Beginning around 1911, in a sparsely populated area of Australia, “Sister” Elizabeth Kenny (1880-1952) developed an empiric approach to rehabilitation following poliomyelitis that combined physical and psychological techniques.
The physical methods she employed included the labor-intensive application of moist warm wraps for muscle spasms, passive range of motion, and massage. Kenny’s physical methods were combined with early mobilization, strong encouragement to achieve both functional independence and a prompt return to normal activities, and confident optimism for improvement. The Kenny methods were widely adopted in the United States and elsewhere in the 1940s (although not in Australia where she was strongly opposed by physicians and, particularly, orthopedic surgeons). Kenny’s approach represented a significant advance in the care of paralyzed patients and helped foster the growth of physical therapy and the medical discipline of physical medicine and rehabilitation.
The first modern intensive care unit was established in Copenhagen in response to the polio epidemic in 1952, based on experience gained using positive pressure ventilation to save hundreds of patients during that epidemic (123; 181).
It was not until the development of the first successful inactivated poliovirus vaccine in the 1950s by American virologist Jonas Salk that the severity of polio epidemics started to decrease. In 1954 and 1955, Salk’s inactivated poliovirus vaccine was successfully tested in a monumental, controlled trial involving more than 1.8 million United States schoolchildren funded by the National Foundation for Infantile Paralysis (131).
Salk's vaccine dramatically curtailed cases of polio in the United States, but one of the difficulties was the required injection.
Various images of children in wheelchairs or wearing leg braces were used to encourage people to support the March of Dimes and to encourage vaccination.
Polish-American virologist Albert Sabin (1906-1993) developed the first effective trivalent live-attenuated (oral) poliovirus vaccine, with somewhat different sources and processes used for component vaccines for poliovirus types 1, 2, and 3 to ensure that each had low neurovirulence (63).
The viruses were replicated either in vivo on monkey (MK) or mouse (Mo) or in vitro. The in vitro cultures used cells from different tissues (cynomolgus monkey kidney cells, rhesus monkey testicular or skin tissues). At the dif...
(Upper panel) Mahoney virus. P1 is the precursor of the capsid proteins; P2 and P3 are the precursors of the noncapsid proteins. The genome segment from nucleotide 743 to 3,832 encodes a polyprotein precursor amino acids 1 to 1...
Sabin's first oral poliovirus vaccine, for use against type 1 polioviruses, was licensed in the United States in 1961. His vaccines for type 2 and type 3 polioviruses were licensed in 1962.
The oral live-attenuated Sabin vaccine was easier to administer and produced longer-lasting immunity than the killed-virus Salk vaccine that had to be administered by injection.
So, by 1962, the Sabin vaccine began replacing the Salk vaccine in the United States and in many other countries.
Aerial view of a long line of people waiting for their polio vaccinations. The line surrounded the city auditorium in San Antonio, Texas. (Source: CDC/Mr. Stafford Smith, 1962. U.S. Centers for Disease Control and Prevention, P...
When necessary, the National Guard distributed polio vaccines to areas experiencing epidemics.
Scene at a Birmingham, Alabama airport, where the Alabama National Guard was preparing to fly polio vaccine to Haleyville during the polio epidemic of 1963. (Source: CDC/Mr. Stafford Smith, 1963. U.S. Centers for Disease Contro...
Public-health promotional campaigns for polio vaccination in the early 1960s used the Communicable Disease Center’s national symbol of public health, the "Wellbee" (Note that the Communicable Disease Center was the original name for the CDC, which changed in 1970 to the Center for Disease Control, and in 1992 to the Centers for Disease Control and Prevention).
This 1963 poster featured what at that time, was the Communicable Disease Center’s (CDC) national symbol of public health, the "Wellbee," who was reminding the public to "be well, be clean, WASH YOUR HANDS." CDC used Wellbee in...
This 1963 poster featured what at that time, was the Communicable Disease Center’s (CDC) national symbol of public health, the "Wellbee," who was reminding the public to "be well, be clean, WASH YOUR HANDS". CDC used Wellbee in...
This 1964 poster featured what at that time, was the Communicable Disease Center’s (CDC) national symbol of public health, the "Wellbee," who was reminding the public to "be well, be clean, WASH YOUR HANDS." CDC used Wellbee in...
|
• More than 90% of poliovirus infections are asymptomatic. | |
|
• Approximately 4% to 8% of infected patients develop symptoms of acute poliomyelitis, which can be separated into two distinct clinical phases: the “minor illness,” with an incubation period of 3 to 7 days, and the “major illness,” with onset of symptoms generally 9 to 12 days after exposure. | |
|
• During the major illness, patients may demonstrate signs of meningeal irritation, asymmetric flaccid weakness, muscle spasms, normal or accentuated muscle stretch reflexes, and hyperesthesias with preserved somatosensation. | |
|
• Paralytic poliomyelitis accounts for only 0.1% to 2.0% of all poliovirus infections and can be divided into three types: spinal, bulbar, and bulbospinal. | |
|
• Chronic poliomyelitis infections have occurred infrequently in children with immunodeficiencies who have received the live oral vaccine. |
More than 90% of poliovirus infections are asymptomatic. Approximately 4% to 8% of infected patients develop symptoms of acute poliomyelitis, which can be separated into two distinct clinical phases: the “minor illness,” with an incubation period of 3 to 7 days, and the “major illness,” with onset of symptoms generally 9 to 12 days after exposure (100). During the minor illness, patients present with constitutional symptoms (eg, fever, headache, nausea and vomiting, coryza, and sore throat), which are typically self-limiting, lasting 1 to 2 days. Major illness is associated with CNS infection, which has been estimated to occur in 0.1% to 1.0% of all poliovirus infections (159; 173).
During the major illness, patients may demonstrate signs of meningeal irritation, asymmetric flaccid weakness, muscle spasms, normal or accentuated muscle stretch reflexes, and hyperesthesias with preserved somatosensation. The major illness includes all forms of CNS disease caused by poliovirus, including aseptic meningitis, polioencephalitis, bulbar polio, and paralytic poliomyelitis. These manifestations may occur alone or in combination. About one third of young children who develop the major illness experience a biphasic illness with symptoms of the minor illness preceding onset of CNS disease (98; 240). The major illness often begins with aseptic meningitis. One third of cases are limited to meningitis without detectable motor neuron impairment, which resolves within 5 to 10 days. Polioencephalitis can occur as well, manifesting as obtundation or agitation, autonomic dysfunction and upper motor neuron signs of spasticity, increased deep tendon reflexes, and Babinski signs. Muscle pains, muscle cramps, fasciculations, and radicular pain rarely occur without paralysis, which they precede by 24 to 48 hours.
Paralytic poliomyelitis accounts for only 0.1% to 2.0% of all poliovirus infections and can be divided into three types: spinal, bulbar, and bulbospinal (159; 99; 164). Muscle weakness is preceded by intense myalgias of the involved limbs and the axial skeleton, and it may be relieved by exercise. The hallmark of paralytic poliomyelitis is asymmetric motor paresis, which ranges from mild weakness of a single extremity to complete quadriplegia and respiratory muscle paralysis.
Dr. Joseph Oren, Chief of the CDC Poliomyelitis Surveillance Unit, was examining the child, who was displaying signs of respiratory weakness. A suction machine was being used, in conjunction with a tracheostomy tube to help in ...
Proximal limb muscles are more involved than distal, and legs are more commonly involved than arms. The reflexes are initially brisk and then become absent over time. The pace of development of the paresis ranges from several hours to several days. Rarely, transverse myelitis with paraparesis, urinary retention, sensory complaints and signs, and autonomic dysfunction may be seen (189; 78). Bulbar poliomyelitis occurs in 5% to 35% of paralytic cases and most commonly affects the ninth and tenth cranial nerves.
Some affected patients develop profound leg weakness that produces severe secondary orthopedic problems, limb deformities, and contractures. Common resulting neuro-orthopedic deformities are pes equinovarus and paralytic pes cavus.
Acquired quadrupedal human gait. In the past, poliomyelitis was the most common cause of an acquired quadrupedal human gait (as distinct from normal developmental or volitional quadrupedal gaits) (134). The most dramatic example was the boy photographed by English American photographer Eadweard Muybridge (1830-1904) and American neurologist Francis Xavier Dercum (1856-1931) in 1885.
The images are from Plate 539 in Muybridge’s Animal Locomotion (Muybridge E. Animal locomotion: an electrophotographic investigation of consecutive phases of animal movements, 1872-1885. Philadelphia: University of Pennsylvania...
Note the accentuated anterior pelvic tilt and lumbar lordosis producing a swayback posture, the marked atrophy of the leg muscles, and the back-kneeing (genu recurvatum). The images are from Plate 539 in Muybridge’s Animal Loco...
In a large series of patients hospitalized with poliomyelitis, 3 of 98 patients (3%) with disturbances of locomotion were noted to be “hand walkers” (among a total of 108 for whom function was recorded) (95). Note that the term “hand walkers” generally denoted those that walked with a quadrupedal gait but very rarely referred to individuals who learned to walk exclusively on their hands. Early 20th-century cases of acquired quadrupedal gaits following poliomyelitis, with photographic documentation of a quadrupedal stance were reported, for example, by Austrian pediatrician and neurologist Julius Zappert (1867-1942), Austrian orthopedist Hans Spitzy (1872-1956), German orthopedic surgeon Oskar Vulpius (1867-1936), and German Swiss neurologist Robert Paul Bing (1878-1956) (249; 180; 219; 106; 30; 134).
Case of Hans Spitzy (1912). "Handwalker" girl, 7 years of age. All of the muscles in both legs, except the iliopsoas of each side, are paralyzed. The child can get around only by crawling. Duration of paralysis was 5 years. (So...
Case of German orthopedist Oskar Vulpius (1915). Boy using a hands-and-knees crawl: infantile spinal paralysis (handwalker) (observation of Professor O Vulpius, Heidelberg). (Source: Ibrahim J. Poliomyelitis anterior acuta [acu...
Case of Swiss neurologist Robert Bing (1921). Boy using a hands-and-feet bear crawl: infantile spinal paralysis. So-called "handwalker" as a result of severe dorsal and lumbar poliomyelitis. Note the marked atrophy in the pelvi...
Case of Austrian pediatrician Heinrich Lehndorff (1908). Poliomyelitis in a 7-year-old child. Severe paralysis of both legs. Flexion contracture in the hip joint, severe lordosis of the lumbar spine. "Hand walkers." [Case] of D...
Vulpius’s case (as illustrated by German physician J Ibrahim in a textbook chapter titled “The organic nervous diseases of childhood”) used hands-and-knees crawling, whereas the cases of Bing and Zappert used hand-and-feet crawling. More recent examples of quadrupedal gaits in adults who survived poliomyelitis as children were identified in various countries during the latter half of the 20th century, most often in Africa and Asia (134).
Chronic poliomyelitis infections have occurred infrequently in children with immunodeficiencies who have received the live oral vaccine (198; 248; 246). Onset usually occurs several months after vaccination, and patients may present with a lower motor neuron paralysis or progressive cerebral dysfunction.
Post-polio syndrome. As many as 20% to 30% of patients who recover from paralytic poliomyelitis experience new onset of slowly progressive muscle weakness, pain, atrophy, and fatigue many years after the acute illness, most commonly 3 to 4 decades after acute illness (117; 139; 140; 51). This is known as post-polio syndrome. Both muscle and joint pain are frequent. Patients with a history of bulbar poliomyelitis are particularly at risk of developing dysphagia as well as sleep-disordered breathing abnormalities.
In a retrospective study of 400 patients with poliomyelitis attending a specialized outpatient clinic in Barcelona, Spain, post-polio syndrome was more frequent in women (58%) (209). The mean age at symptom onset was 52 years and was earlier in women. Common problems included pain (85%), loss of strength (40%), fatigue (66%), cold intolerance (20%), dysphagia (12%), depressive symptoms (32%), and cognitive complaints (9%). Fatigue or tiredness, depression, and cognitive complaints were significantly more frequent in women. Electromyographic findings suggestive of post-polio syndrome were reported in 59%.
Morbidity and mortality in poliomyelitis vary depending on the severity of illness. Although a minor illness typically causes little or no sequelae, major illness can lead to muscle paralysis, respiratory arrest, and death. Case fatality is 1.5% to 6.7%, assuming availability of optimal care (67). A study on a particularly virulent outbreak of wild poliovirus in the Republic of Congo found up to 92% of cases had residual paralysis 60 days after disease onset (185). Efficient surveillance and proactive case management may improve morbidity and mortality. Complications of poliomyelitis include respiratory distress often necessitating mechanical ventilation, dysphagia, and pneumonia (77; 80). Prolonged immobility may lead to decubitus ulcers and limb contractures (80). Provocative paralysis (which can occur following intramuscular injection) should also be anticipated when managing poliomyelitis.
Orthopedic issues include hip, back, and extremity pain; muscle strain; ligament sprain; spondylosis; kyphoscoliosis; exaggerated lumbar lordosis; genu recurvatum; and other deformities (54; 80; 225).
In the past, poliomyelitis was probably the most common cause of an acquired quadrupedal human gait (as opposed to normal developmental or volitional quadrupedal gaits) (132; 133; 134).
Postpoliomyelitis patients transitioning to adulthood have a high frequency of falls, osteoporosis, and fractures (217). Bone mineral density tends to be worse in the worst-affected leg (217). In a retrospective analysis of 204 postpoliomyelitis patients, recurrent falls were documented in 39% of patients and osteoporosis in 21%. Not surprisingly, osteoporosis was more frequent in women and patients with fractures. At least one fracture occurred in half (52%) of the patients and more than one fracture occurred in 40% of the patients. The median age for the first fracture was 58 years (range, 30 to 83 years) and most fractures occurred in the affected limb (73%).
Sleep disturbances also occur more frequently and are most commonly associated with the bulbar variant of poliomyelitis (54; 75; 13).
Physical disabilities experienced often lead to emotional symptoms (80). Psychotherapy, group rehabilitation, regular follow-ups, and patient education methods can be used to improve mental status and overall well-being (75).
Patients with post-polio syndrome develop generalized fatigue, muscle weakness, atrophy, pain, fasciculations, cramps, and cold intolerance occurring several years (usually more than 15 years) after the acute episode (93; 54; 117; 139; 140; 51).
A 10-year-old boy living in Nigeria was brought to the pediatric emergency department for evaluation of a 2-day history of malaise and diarrhea, with both an increased frequency of stooling and the passage of watery, non-bloody stools. On examination, he was febrile with a temperature of 38.5 degrees Celsius. His abdomen was non-tender, and he had no palpable organomegaly. On neurologic examination, he was conscious and fully oriented but lethargic with mild neck stiffness. He had no cranial nerve palsy. Muscle tone was mildly reduced in the right leg compared to the left. Power was grade 4/5 on the Medical Research Council scale in the right ankle and 5/5 in all other muscle groups. Deep tendon reflexes were grade 1 at the right ankle and grade 2 at the other sites. Plantar response was flexor bilaterally. Sensory examination was unremarkable. Complete blood count, erythrocyte sedimentation rate, electrolytes, and stool (for microscopy, culture, and sensitivity) were ordered.
The following day, his motor strength was reduced to 3/5 in the right leg, and within 48 hours, it was 1/5 and 4/5 on the right and left legs, respectively. Blood count was unremarkable. CSF analysis revealed a mild increase in leukocytes (18 cells/µl) and protein (45 mg/dl). CSF glucose was normal (70 mg/dl). Stool was negative for pathogenic bacteria or blood. Stool samples were positive for wild-type poliovirus 1. He was maintained on bed rest, and specific instruction was given to be vigilant for respiratory distress and to avoid intramuscular injections.
Further interview with the mother revealed that the boy did not complete his polio immunizations as he had only the oral polio vaccine 1 at 6 weeks after birth but did not receive the second and third oral polio vaccine doses in the subsequent months. She could not remember if he had any booster doses at 18 months or later. Three weeks later, power was 2/5 on the right and 4/5 on the left legs.
|
• Poliomyelitis is an infection caused by the polioviruses, which are human enteroviruses. | |
|
• Poliovirus, as with other enteroviruses, is most commonly spread by fecal-hand-oral transmission. | |
|
• Although the precise cause of post-polio syndrome is unknown, the cause is probably related to overstress or exhaustion of the residual surviving motor units following acute poliovirus infection. |
Poliomyelitis is an infection caused by the polioviruses, which are human enteroviruses. Three poliovirus serotypes are distinguished from one another by type-specific antisera (09). Before the introduction of poliovirus vaccines, naturally occurring polioviruses circulated in temperate climates with a marked seasonal variation resulting in peak activity between July and October and low levels between December and May. Poliovirus type 1 was responsible for 80% to 90% of all epidemics of paralytic poliomyelitis in the United States (27; 173).
Poliovirus is a nearly spherical icosahedral-shaped virus, as revealed by x-ray crystallography and electron microscopy.
(Source: Filman DJ and Hogle JM (1989) [https://www.rcsb.org/structure...]. Creative Commons Attribution-Share Alike 4.0 International [CC BY 4.0] license, creativecommons.org/l...
Scanning electron microscopic (SEM) image depicted a number of round, polio virions, or virus particles, scattered on a background plane. (Source: CDC. U.S. Centers for Disease Control and Prevention, Public Health Image Librar...
This transmission electron microscopic image reveals ultrastructural features exhibited by numerous icosahedral-shaped poliovirus particles. (Source: CDC/ Dr. Fred Murphy, Sylvia Whitfield (1975). U.S. Centers for Disease Contr...
The poliovirus genome consists of a single-stranded, positive-sense polarity RNA molecule, which encodes a single polyprotein (61).
This genomic region has been divided into six domains (I to VI), of which domain I constitutes the cloverleaf and the remaining domains comprise the internal ribosome entry site (IRES). Spacer sequences without complex secondar...
The 5' non-translated region (NTR) harbors two functional domains, the cloverleaf and the internal ribosome entry site (IRES). The 5' NTR is covalently linked to the viral protein VPg.
The cellular life cycle of poliovirus is somewhat complicated (39; 61).
First, poliovirus binds to the cell surface macromolecule CD155, which functions as the virus receptor (61). Viral RNA uncoating is mediated by receptor-dependent conformational changes and destabilization of the virus capsid (39; 61).
The particles are then internalized by an actin- and tyrosine kinase-dependent, but clathrin- and caveolin-independent, mechanism (39). The release of the viral genome takes place only after internalization from an endocytotic compartment localized within 100 to 200 nm of the plasma membrane (39). On release of the RNA genome, the empty capsid is transported away along microtubules (39). Translation of the viral RNA is mediated by internal ribosome entry site (IRES), an RNA element that allows for translation initiation in a cap-independent manner, as part of protein synthesis. Proteolytic processing of the viral polyprotein produces mature structural and nonstructural proteins. The positive-sense RNA serves as the template for complementary negative-strand synthesis. Newly synthesized positive-sense RNA molecules can either serve as templates for translation or associate with capsid precursors to undergo encapsidation, which ultimately generates progeny virions. Lysis of the infected cell results in release of infectious progeny virions.
Poliovirus, as with other enteroviruses, is commonly spread by fecal-hand-oral transmission (63).
Major risk factors for poliovirus transmission include poor sanitation and hygiene conditions, high population density, and tropical or subtropical climate. (A) Polioviruses infect humans by the fecal-oral route. This acid-resi...
After ingestion, the poliovirus implants in the oropharynx and small bowel and penetrates the mucosa via specialized microfold cells and other epithelial cells overlying submucosal lymphoid tissues (99; 182; 177). After replication in the submucosal lymphatic tissue, the virus spreads to the lymph nodes, and then to the bloodstream (32). In a minority of infections, further replication of virus in reticuloendothelial tissues leads to a major viremia. CNS invasion is much more likely to occur during secondary viremia but can infrequently occur during primary viremia. As demonstrated in experimental studies in primates, tonsils, mesentery lymph nodes, and intestinal mucosa are major target sites of viral replication (216). Early in the infectious process, poliovirus replication occurs in both epithelial cells (which accounts for virus shedding in the gastrointestinal tract) and lymphoid/monocytic cells in tonsils and Peyer patches (which accounts for viremia) (216). Genetic variants in innate immune defenses and cell death pathways may contribute to the clinical presentation of poliovirus infection (12).
The exact route through which poliovirus enters the CNS is unclear (170; 171; 172; 177). There is some suggestion based on animal models that viral replication in skeletal muscles precedes transport of poliovirus to the spinal cord via the peripheral nerve (36). The anterior horn cells and other motor neurons are selectively vulnerable to poliovirus infection (33; 112; 94; 56). Mitochondria are important in poliovirus-induced apoptosis and mitochondrial dysfunction results from an imbalance between pro- and anti-apoptotic pathways (31). Pathologic changes occur within 1 to 2 days after poliovirus infects the neuron, and an inflammatory response ensues with meningeal, perivascular, and parenchymal infiltrates (35; 34; 110; 245). Neuronal destruction by lytic replication of the poliovirus causes paralytic poliomyelitis in less than 1% of those infected (177). Individuals who have had polio may experience denervation with subsequent reinnervation for many years. Many have spotty loss of anterior horn motor neurons, and the anterior horn may be shrunken and sclerotic in the chronic stage.
Photomicrograph of a spinal cord tissue sample at low magnification in the region of the anterior horn, revealing polio type III degenerative changes. (Source: CDC/Dr. Karp, Emory University, 1964. U.S. Centers for Disease Cont...
Photomicrograph of a spinal cord tissue sample at higher magnification in the region of the anterior horn, revealing polio type III degenerative changes. (Source: CDC/Dr. Karp, Emory University, 1964. U.S. Centers for Disease C...
Although the precise cause of post-polio syndrome is unknown, the cause is probably related to overstress or exhaustion of the residual surviving motor units following acute poliovirus infection. Terminal elements of surviving alpha motor neurons that sprout to reinnervate adjacent myofibrils following initial infection are believed to become overstressed (91; 04; 183; 215; 124). Muscle fatigue in post-polio syndrome may be due to impaired activation beyond the muscle membrane at the level of excitation-contraction coupling or may have a central origin. Other possible mechanisms have been considered, including immunologic events and other virus-host interactions (60), but some studies have found no evidence of a dysimmune condition (124).
Vaccine-derived polioviruses and vaccine-associated paralytic poliomyelitis. Vaccine-derived poliovirus (VDPV) results from the use of oral poliovirus vaccine in low- and middle-income countries. VDPVs are rare strains of poliovirus that have genetically mutated from the attenuated strains contained in the oral polio vaccine. Normally, when a child is vaccinated, the weakened vaccine strains replicate in the intestine and enter the bloodstream, triggering a protective immune response. Rarely, during this replication process, some vaccine virus may genetically mutate from the original attenuated strain, become neurovirulent, and cause vaccine-associated paralytic poliomyelitis. In addition, like infections with wild polioviruses, vaccinated children typically excrete the vaccine strains of poliovirus for 6 to 8 weeks. If a population is under-immunized, there may be enough susceptible children for the excreted attenuated poliovirus to begin circulating in the community; with uninterrupted recirculation, the attenuated vaccine strain may reacquire neurovirulence, at which time these are called circulating vaccine-derived polioviruses (cVDPV). Prolonged replication of VDPV has also been observed in people with immune deficiency disorders. In the absence of an adequate immune response, such individuals may excrete immunodeficiency-related VDPVs (or iVDPVs) for prolonged periods. Most patients with iVDPVs either stop excretion within 6 months or die. Finally, ambiguous VDPVs (aVDPVs) are either isolated from people with no known immunodeficiency or isolated from sewage whose ultimate source is unknown. As poliovirus serotypes are eliminated, it is necessary to continue to shift away from the use of live attenuated vaccines to minimize the risk of VDPV.
Therefore, there are three categories of VDPV:
(1) Circulating VDPVs (cVDPVs): VDPV for which there is evidence of human-to-human transmission in the community. Isolates must either be from (1) at least two individuals (not necessarily cases of acute flaccid paralysis) who are not direct contacts; (2) from one individual plus environmental samples; or (3) from at least two environmental samples collected more than 2 months apart or from more than one distinct collection site. Low levels of population immunity to polio (due to low vaccination coverage) increase the risk of cVDPV (07; 08).
(2) Immunodeficiency-associated VDPVs (iVDPVs): VDPV isolated from patients with primary immunodeficiencies. Patients with iVDPV are potential poliovirus reservoirs that can potentially reintroduce polioviruses into a community in the post-eradication era. Roughly a third of patients with iVDPVs have never had paralysis (212).
(3) Ambiguous VDPVs (aVDPVs): VDPV isolates (human or environmental) without evidence of circulation and from individuals with no known immunodeficiency. These may subsequently be reclassified as “circulating” if genetically linked isolates are identified.
|
• There are now three countries with endemic poliomyelitis—Afghanistan, Pakistan, and Papua New Guinea—and there are major threats to increasing polio outbreaks due to lack of accessibility to polio vaccines in these regions and neighboring areas. | |
|
• Among the three wild poliovirus serotypes, only type 1 has been reported in polio cases or detected from environmental surveillance globally since 2012. | |
|
• Type 1 continues to be isolated regularly from environmental surveillance sites in Pakistan and Afghanistan, signifying persistent transmission. | |
|
• Vaccine-derived polioviruses (VDPVs) are rare strains of poliovirus that have genetically mutated from the attenuated strains contained in the oral polio vaccine. | |
|
• If a population is under-immunized, there may be sufficient susceptible children for the excreted VDPVs to begin circulating in the community; with sufficient time of uninterrupted recirculation, vaccine-derived poliovirus may reacquire neurovirulence and are then called circulating vaccine-derived polioviruses (cVDPV). | |
|
• Prolonged replication of vaccine-derived polioviruses has also been observed in people with immune deficiency disorders; in the absence of an adequate immune response, such individuals may excrete immunodeficiency-related vaccine-derived polioviruses (or iVDPVs) for prolonged periods. | |
|
• Ambiguous vaccine-derived poliovirus (aVDPV) are vaccine-derived polioviruses that are either isolated from people with no known immunodeficiency or isolated from sewage whose ultimate source is unknown. | |
|
• As poliovirus serotypes are eliminated, it is necessary to continue to shift away from use of live attenuated vaccines to minimize the risk of vaccine-derived poliovirus cases. |
Accurate prevalence figures estimating the number of survivors of poliomyelitis are not available (109).
Global Polio Eradication Initiative (GPEI) and optimal poliovirus vaccines. The Global Polio Eradication Initiative (GPEI), launched in 1988, represents the single largest, internationally coordinated public health project to date. GPEI is a public-private partnership led by national governments with six core partners: the World Health Organization (WHO), Rotary International, the U.S. Centers for Disease Control and Prevention (CDC), the United Nations Children’s Fund (UNICEF), the Bill & Melinda Gates Foundation, and Gavi, the Vaccine Alliance. Its goal is to eradicate polio worldwide. Although controlling polio can be less costly in the short term, the cumulative costs of controlling polio will overtake those of eradication (205).
(Source: Dattani S, Spooner F, Ochmann S, Roser M. Polio. First published in November 2017, and last updated in May 2024. Available at: ourworldindata.org/polio. Accessed May 2024. Creative Commons Attribution 4.0 International...
Controlling the disease can have lower costs in the short term, but the cumulative costs can quickly overtake those of eradication. (Source: Roser M, Ochmann S, Behrens H, Ritchie H, Dabonaite B. Eradication of diseases: which ...
To understand the discussion of polio trends and current activity in different WHO regions, it is necessary to have some familiarity with how the WHO categorizes different world regions.
The complexities of war, poverty, and pandemic (COVID-19) have all complicated the efforts of GPEI and triggered debate on the economic return of polio eradication, as well as financing, feasibility, and strategy (23; 16; 18; 17; 68; 69; 197; 105; 14; 228; 227; 229). During the COVID-19 pandemic, immunization programs in many countries experienced setbacks (162; 204; 208; 66). Marked differences in the reporting of poliomyelitis cases in underdeveloped countries were initially quite large, forcing adjustments for underreporting to generate estimates of actual disease incidence (68). Economic analyses have found strong economic justification for the GPEI despite the rising costs of the initiative; the positive net benefits of the GPEI remain robust over a wide range of assumptions (68).
The "end game" will also depend on the vaccines used in different countries. The standard vaccines have been the inactivated polio vaccine (IPV; Salk) and the attenuated oral polio vaccine (OPV; Sabin). Unfortunately, IPV is associated with various logistical problems that make it less suitable in poorly developed countries, including its minimal intestinal mucosal immunogenicity, its relatively high cost, and various operational issues (161). Many countries have switched entirely to IPV, but Asia, Africa, and major areas of Oceana and South America continue to use a combination of IPV and OPV (142; 224).
OPV, comprised of live attenuated Sabin strains for each type of poliovirus, was the GPEI vaccine of choice due to its ease of use, low cost, and ability to induce intestinal mucosal immunity, which is required for preventing transmission (224). The problem with OPV is that it continues to cause vaccine-associated and vaccine-derived paralytic poliomyelitis (161; 224).
The GPEI used exclusively trivalent OPV (tOPV) until 2005, when monovalent oral poliovirus vaccine type 1 (mOPV1) and type 3 (mOPV3) were licensed. The number of polio-endemic countries decreased from more than 125 in 1988 to four after Egypt and Niger interrupted endemic transmission in 2005 (Afghanistan, India, Pakistan, Nigeria) (86; 224). Despite this success, the eradication effort must shift away from OPV because the genetic instability of the Sabin strains allows them to revert to neurovirulent forms causing vaccine-derived poliovirus outbreaks. Prolonged replication of the OPV strains (eg, in a B-cell deficient vaccinee or circulation in an under-vaccinated population) allows mutations and recombinations that can result in the emergence of new polioviruses as neurovirulent and transmissible as wild poliovirus (eg, after losing all attenuation mutations in the original vaccine) (41). Consequently, continued use of Sabin strains could negate the achievements of the eradication effort.
In November 2012, the Strategic Advisory Group of Experts (SAGE) on Immunization endorsed the "Strategic Plan of Action for Polio Eradication 2013–2018" that called for the withdrawal of Sabin strains from the OPV, starting with Sabin type 2 (OPV2) (88). Indigenous wild poliovirus type 2 (WPV2) was last detected in 1999 in Northern India, and OPV2 use continues to cause outbreaks of vaccine-associated paralytic poliomyelitis (VAPP), particularly in populations with suboptimal immunity (188).
In 2013, the GPEI recommended that at least one dose of IPV be introduced into routine immunization programs in advance of OPV2 withdrawal in April 2016 (88; 87; 230). A single IPV dose administered at 14 weeks of age or older is highly immunogenic, inducing nearly universal type 2 immunity (seroconversion and priming), with immunity persisting for at least 28 months (214). Although most countries successfully introduced IPV into routine immunization, more than 43 million children did not receive IPV after OPV2 cessation due to supply constraints (224).
Supply constraints also limited the use of IPV in response to cVDPV2 outbreaks, so in 2017 use of IPV was no longer recommended for this purpose (224). After IPV supply constraints were finally addressed, the GPEI adopted a two-dose IPV schedule for routine immunization in 2020, in addition to catch-up IPV vaccinations for children who had not received IPV after OPV2 cessation (World Health Organization 2020). In 2023, the SAGE restored the recommendation for IPV use in cVDPV2 outbreaks.
The switch to a bivalent OPV was intended to reduce VDPV outbreaks, but this did not happen (202). In Africa, cVDPV2 outbreaks escalated markedly as an increasing number of children who were no longer receiving the type 2 virus in routine immunization became susceptible to it (142; 202; 224).
The monovalent (mOPV2) vaccination campaigns launched in response to cVDPV2 outbreaks were too limited geographically, came too late, and didn’t reach enough children (202; 224).
After the global withdrawal of OPV2 from routine immunization in 2016, outbreak response use of OPV2 has contributed to a cycle of VDPV2 emergences that threaten eradication (89). Risk factors for vaccine-derived poliovirus emergences in Africa from 2016 to 2019 included low population immunity and limited campaign size for outbreak response: a 10–percentage point increase in population immunity in children younger than 5 years corresponded to an 18% decrease in per-campaign relative risk (89). Because of the withdrawal of OPV2 and the limitations of outbreak response, the number of cVDPV2 cases increased about 10-fold since 2015 (142; 202). Paralytic polio cases caused by type 2 vaccine-derived polioviruses now exceed 3000 cases based on reports from 39 countries (115). Based on prior polio endgame modeling and assuming current national and GPEI outbreak response performance, there is now no probability of successfully eradicating type 2 polioviruses in the near future, regardless of vaccine choice; moreover, worst-case scenarios could result in rapid expansion of paralytic serotype 2 cases and preclude the goal of permanently eradicating poliomyelitis in the foreseeable future (115).
Plans for cessation of bivalent OPV (bOPV, containing OPV types 1 and 3) remain undefined, and options for bOPV cessation are unlikely to succeed without a strategy of bOPV intensification to increase population immunity prior to cessation (114).
It is hoped that newer polio vaccines will obviate some of the problems of the Salk and Sabin vaccines, making them better choices to finalize disease eradication (201; 161; 15). In a campaign in The Gambia, in West Africa, novel oral polio vaccine type 2 (nOPV2) was well tolerated and safe, with high rates of seroconversion (poliovirus type 2 seroconversion rates were 70% to 86% following one dose of nOPV2 and 91% to 95% following two doses) (24; 179). In a comparison of the effectiveness of nOPV2 against cVDPV2 paralysis with that of mOPV2 and IPV in Nigeria, there was no significant difference in estimated effectiveness of the two oral vaccines, supporting the recommendation that the more genetically stable nOPV2 should be preferred in cVDPV2 outbreak response (53). However, in Liberia, there was an unexpectedly low type 2 seroconversion rate after two reported doses of nOPV2 and no significant difference between type 2 seroprevalence in children aged 6 months or older who were reported to have received two doses of nOPV2 (42%), one dose (28%), or no doses (38%) (118). Co-administration of nOPV2 and bOPV blunted immunogenicity for poliovirus type 2, but not for types 1 and 3, a major drawback of using co-administration as a vaccination strategy (242).
|
Endgame issues |
IPV (Salk) |
OPV (Sabin) | |
|
Immunologic | |||
|
Primary intestinal mucosal immunogenicity |
Minimal |
Present | |
|
Operational | |||
|
SIA use for outbreak response |
Difficult |
Suitable | |
|
Production risks for containment failure |
High |
Low | |
|
Cost |
High |
Low | |
|
Adverse events | |||
|
Risk of VAPP |
None |
Present | |
|
Risk of VDPVs |
None |
Present | |
|
Abbreviations: IPV, inactivated poliovirus vaccine; OPV, oral poliovirus vaccine; SIA, supplementary immunization activity; VAPP, vaccine-associated paralytic poliomyelitis; VDPD, vaccine-derived poliovirus | |||
|
| |||
|
Vaccine type |
Attribute |
Examples |
Status |
|
Parenteral, inactivated |
Dose sparing by fractional dose administration |
Intradermal |
Adopted in several countries |
|
Intramuscular |
Clinical studies in progress | ||
|
Dose sparing by adjuvantation |
Alum (AJ Vaccines) |
Licensed in 2019, WHO prequalified 2020 | |
|
Adjuvanted for mucosal immunity |
dmLT-IPV |
In clinical trial | |
|
Safer production |
Sabin IPV |
Licensed in Japan, China, WHO prequalified 2020 | |
|
S19 IPV |
In clinical development | ||
|
Virus-like particles |
Preclinical | ||
|
Oral, live attenuated |
Enhanced genetic stability |
nOPV vaccine candidates |
nOPV granted emergency use authorization by WHO; nOPV1 and nOPV3 in preclinical development |
|
| |||
|
Source: (161) | |||
Vaccine choice for outbreak response. Due to its cost, ease-of-use, and ability to induce mucosal immunity, OPV is more cost-effective as an outbreak control measure in countries in which OPV remains in use (231). Using IPV for outbreak response will likely only stop outbreaks for polioviruses of relatively low transmission potential in countries with very high overall immunization coverage and seasonal transmission dynamics.
Surveillance. Poliovirus transmission is primarily detected through syndromic surveillance for acute flaccid paralysis among children younger than 15 years old, with confirmation by laboratory testing of stool specimens (222; 122).
Abbreviations: AFP, acute flaccid paralysis; NPAFP, nonpolio acute flaccid paralysis; WHO, World Health Organization.
Notes:
(1) Targets: two or more NPAFP cases per 100,000 persons younger than 15 years of age per ...
Abbreviations: AFP, acute flaccid paralysis; NPAFP, nonpolio acute flaccid paralysis; WHO, World Health Organization.
Notes:
(1) The number of NPAFP cases per 100,000 children younger than 15 years of age per year....
Public awareness. Public health messaging in native languages with images of polio victims is used to increase public awareness of the problem and the availability of protective vaccines.
This 2000 photograph depicted a sign displayed in Gorakhpur, India, which advertised a Pulse Polio Vaccination Program that had been scheduled to take place on February 27, 2000, the main purpose of which was to vaccinate child...
Polio worldwide. Polio cases have decreased by over 99% since 1988, from an estimated 270,000 to 350,000 cases in more than 125 endemic countries to 694 reported cases due to wild-type virus in 2021 (six due to wild-type poliovirus type 1 from Afghanistan, Pakistan, and Malawi and 688 due to cVDPV, 672 due to cVDPV2, and 16 due to cVDPV1) (68; 74; 81; 156; 157; 169; 84; 102; 193; 58). Reported poliomyelitis incidence declined by roughly 2 orders of magnitude between the mid-1980s and the mid-2000s, which taking account of the adjustments needed for underreporting, is less dramatic than the nearly 3 orders-of-magnitude decrease in estimated decline in incidence (68; 58).
Shown is the "base case" reported by Duintjer Tebbens and colleagues. (Sources: Dattani S, Spooner F, Ochmann S, Roser M. Polio. Published online at OurWorldInData.org 2017, last revised 2022. Retrieved from: https://ourworldin...
Reported cases of polio underestimate actual cases because people with acute flaccid paralysis may not be detected and tested for poliovirus in time. (Source: Dattani S, Spooner F, Ochmann S, Roser M. Polio. Published online at...
The result is that an estimated 2.2 million cases of paralytic polio were averted between 1988 and 2018 (68; 57). The WHO estimates are somewhat greater (161).
With the Global Polio Eradication Initiative (GPEI), 105 countries received support for surveillance, childhood immunizations, and campaigns to control outbreaks. (Sources: Thompson KM, Kalkowska DA. An updated economic analysi...
As more countries have progressively become polio-free, eradication efforts have been more focused on the Eastern Mediterranean WHO regions (57). Unfortunately, some areas free from wild-type poliovirus have maintained insufficient vaccine coverage to ensure that they remain polio-free.
Annual number of reported cases of paralytic polio per million people. This includes all reported cases from wild polioviruses and vaccine-derived polioviruses. (Source: Dattani S, Spooner F, Ochmann S, Roser M. Polio. First pu...
The CDC and WHO have collected data concerning worldwide polio by country that show the progress, and the limitations of progress, in incident cases of poliomyelitis from 2012 through 2023 (167; 190; 166; 65; 120; 90; 29; 193; 137; 222; 83; 122). In that interval, Afghanistan, Pakistan, and Nigeria have contributed the most cases, with scattered additional cases, particularly in African countries (58).
Data as of May 20, 2014. (Source: Moturi EK, Porter KA, Wassilak SG, et al. Progress toward polio eradication--worldwide, 2013-2014. MMWR Morb Mortal Wkly Rep 2014;63[21]:468-72.)
Other countries include Cameroon (n = 5), Equatorial Guinea (n = 5), Ethiopia (n = 1), Iraq (n = 2), Somalia (n = 5), and Syria (n = 1). (Source: Morales M, Tangermann RH, Wassilak SG. Progress toward polio eradication - worldw...
Data as of May 3, 2019. (Source: Greene SA, Ahmed J, Datta SD, et al. Progress toward polio eradication - worldwide, January 2017-March 2019. MMWR Morb Mortal Wkly Rep 2019;68[20]:458-62.)
Data as of August 3, 2021. Abbreviation: WPV1 = wild poliovirus type 1. (Source: Bigouette JP, Wilkinson AL, Tallis G, Burns CC, Wassilak SGF, Vertefeuille JF. Progress toward polio eradication - worldwide, January 2019-June 20...
Data as of May 5, 2022. Abbreviation: WPV1 = wild poliovirus type 1. (Source: Rachlin A, Patel JC, Burns CC, et al. Progress toward polio eradication - worldwide, January 2020-April 2022. MMWR Morb Mortal Wkly Rep 2022;71[19]:6...
This includes reported cases of paralytic polio from any wild poliovirus strain (WPV1, WPV2, WPV3). As can be seen, the predominant type of circulating vaccine-derived poliovirus (cVDPV) was type 2 (cVDPV2). (Source: Dattani S,...
Data current as of May 5, 2023. Abbreviation: WPV1, wild poliovirus type 1. (Source: Lee SE, Greene SA, Burns CC, Tallis G, Wassilak SG, Bolu O. Progress toward poliomyelitis eradication - worldwide, January 2021-March 2023. MM...
Abbreviation: WPV1, wild poliovirus type 1. (Source: Geiger K, Stehling-Ariza T, Bigouette JP, et al. Progress toward poliomyelitis eradication - Worldwide, January 2022-December 2023. MMWR Morb Mortal Wkly Rep 2024;73[19]:441-...
Due to the GPEI, poliomyelitis due to wild-type virus has now been eliminated from the Americas, Europe, and the Western Pacific, whereas cases in Africa and Asia have markedly decreased (45; 46). In 1994, the WHO Region of the Americas was certified polio-free, followed by the WHO Western Pacific Region in 2000 and the WHO European Region in June 2002. In 2014, the WHO Southeast Asia Region was certified polio-free, meaning that transmission of wild poliovirus has been interrupted in 11 countries stretching from Indonesia to India.
Eradication of wild poliovirus types 2 and 3 and persistence of wild poliovirus 1 circulation. Among the three wild poliovirus serotypes, only type 1 has been reported in polio cases or detected from environmental surveillance globally since 2012 (103; 101; 148; 150; 58).
Type 1 continues to be regularly isolated from environmental surveillance sites in Pakistan and Afghanistan, signifying persistent transmission (103; 101; 148; 150; 193).
Indigenous wild poliovirus type 2 (WPV2) was last isolated in October 1999 in India (44). The Global Commission for the Certification of Poliomyelitis Eradication concluded in September 2015 that indigenous WPV2 has been eradicated worldwide.
Type 3 wild poliovirus was declared eradicated in October 2019. It was last detected in northern Nigeria in November 2012.
In 2022, Afghanistan and Pakistan reported 22 WPV1 cases, compared with five in 2021; as of May 5, 2023, a single WPV1 case was reported in Pakistan in 2023 (137).
Abbreviation: WPV1 = wild poliovirus type 1. (Source: Lee SE, Greene SA, Burns CC, Tallis G, Wassilak SG, Bolu O. Progress toward poliomyelitis eradication--worldwide, January 2021-March 2023. MMWR Morb Mortal Wkly Rep 2023;72[...
A WPV1 case was reported on the African continent for the first time since 2016, when officials in Malawi confirmed WPV1 in a child with paralysis onset in November 2021; neighboring Mozambique subsequently reported eight genetically linked cases (137).
Vaccine-derived polioviruses. Circulating vaccine-derived poliovirus (cVDPV) outbreaks typically occur when transmission of Sabin strain poliovirus is prolonged in underimmunized populations, allowing viral genetic reversion to neurovirulence (07; 08).
From 2016 to 2023, cVDPV2 (ie, cVDPV caused by poliovirus type 2) was the predominant strain (95%) among the 1818 human cVDPV cases reported worldwide (126; 58).
New cVDPV2 outbreaks were often linked to poor coverage with monovalent Sabin oral poliovirus vaccine type 2 during outbreak response campaigns. Of 40 countries reporting cVDPV cases or isolates, more than half (55%) had polio vaccination coverage below 80%. Low vaccination coverage was associated with increased odds of reporting cVDPV after adjusting for confounding effects of gross domestic product per capita, female adult literacy rate, maternal mortality rate, and the Global Peace Index (Adjusted OR = 83; 95% CI: 5-1388).
Data as of March 24 to 27, 2020. Abbreviations: cVDPV1 = cVDPV type 1; cVDPV2 = cVDPV type 2. (Source: Alleman MM, Jorba J, Greene SA, et al. Update on vaccine-derived poliovirus outbreaks--worldwide, July 2019 to February 2020...
Data as of November 9, 2021. Abbreviations: cVDPV = circulating vaccine-derived poliovirus; cVDPV1 = cVDPV type 1; cVDPV2 = cVDPV type 2; cVDPV3 = cVDPV type 3. (Source: Alleman MM, Jorba J, Greene SA, et al. Update on vaccine-...
Courtesy of Ondrej Mach and Ajay Kumar Goel, World Health Organization. Abbreviations: AFP, acute flaccid paralysis; bOPV, bivalent oral poliovirus vaccine; cVDPV, circulating vaccine-derived poliomyelitis; ES, environmental su...
The number of cVDPV2 cases increased from 366 in 2019 to 1078 in 2020 (08). From January 2020 to June 2021, there were 38 cVDPV2 emergences in active transmission in 34 countries; 28 (82%) of these countries are in Africa. Of these 38 cVDPV2 emergencies, 50% were previously detected from 2017 to 2019, 8% were newly detected in 2019, and 42% were newly detected from 2020 to 2021 (08). During the reporting period, 15 (58%) of the 26 emergencies in active transmission in African countries were detected, either in patients with acute flaccid paralysis or through environmental surveillance (of sewage), outside of the country of first isolation of genetically linked virus. cVDPV2 is now the predominant type of cVDPV (58; 63; 142).
Some cases of paralytic polio arise from vaccine-derived strains that have reverted to a form that can cause disease. There are three vaccine-derived strains of paralytic polio: VDPV1, 2, and 3. This shows cases of circulating ...
Data derived from WHO HQ as of February 27, 2024. Abbreviations: AFP, acute flaccid paralysis; cVDPV, circulating vaccine-derived poliovirus; WPV, wild-type poliovirus. (Source: Lopez Cavestany R, Eisenhawer M, Diop OM, Verma H...
From January 2020 to June 2021, cVDPV1 was detected in Malaysia, Madagascar, and Yemen (08).
Note that in 2017 the graphs for wild-type type 1 and cVDPV cross over so that cVDPV exceeds the number of wild-type cases. (Figure prepared by Dr. Douglas J Lanska from data from the Centers for Disease Control and Prevention ...
A total of 859 cVDPV cases occurred during 2022, an increase of 23% from 698 cases in 2021 (Lee et a 2023). cVDPVs were detected in areas where poliovirus transmission had long been eliminated (including in Canada, Israel, the United Kingdom, and the United States) (137). In addition, cocirculation of multiple poliovirus types occurred in multiple countries globally (including the Democratic Republic of the Congo, Israel, Malawi, Mozambique, Republic of the Congo, and Yemen) (137).
In 2023, countries reporting cases of paralytic poliomyelitis from vaccine-derived viruses included multiple countries in Western, Central, and Eastern Africa, Madagascar, Yemen, and Indonesia (58; 142).
Clinical acute flaccid paralysis (AFP) and environmental surveillance (ES) poliovirus detections are depicted. On the left, there is a per-country list of the latest detections of AFP and ES. Data source: from WHO HQ as of Febr...
WHO Region of the Americas: free of wild-type poliovirus (1994). The WHO Region of the Americas was declared polio-free of wild-type poliovirus in 1994, the first of the six WHO regions to achieve this designation.
United States. In the United States, polio generally occurred in small outbreaks during the 19th century but often occurred in large epidemics in the first half of the 20th century. By the mid-20th century, polio occurred throughout the U.S., but often in relatively localized epidemics or outbreaks (59). The advent of the Salk vaccine, which was adopted throughout the U.S. in April 1955, dramatically curtailed polio in this country. The last case of polio caused by wild poliovirus in the U.S. occurred in 1979.
The reported figures include both wild- and vaccine-derived type polio infections that occurred indigenously and as imported cases. (Source: Dattani S, Spooner F, Ochmann S, Roser M. Polio. First published in November 2017, and...
In 2013, a case of vaccine-associated paralytic poliomyelitis was identified in an immigrant child with severe combined immunodeficiency syndrome in Texas (233).
In July 2022, an unvaccinated immunocompetent young adult from Rockland County, New York, who was experiencing acute flaccid weakness was diagnosed with polio (138; 135). The patient was hospitalized with acute flaccid myelitis after presenting with fever, neck stiffness, gastrointestinal symptoms, and limb weakness. Vaccine-derived poliovirus type 2 (VDPV2) was detected in stool specimens obtained on days 11 and 12 after initial symptom onset. Related Sabin-like type 2 polioviruses were detected in wastewater (sewage) in the patient’s county of residence and in neighboring Orange County up to 25 days before and 41 days after the patient’s symptom onset. The earlier samples had been obtained for SARS-CoV-2 wastewater monitoring.
Abbreviations: ED = emergency department; VDPV2 = type 2 vaccine-derived poliovirus. Wastewater, also referred to as sewage, includes water from household or building use (eg, toilets, showers, and sinks) that can contain human...
Poliovirus testing of 1076 wastewater samples collected from March 9 to October 11, 2022, from 48 sewersheds serving parts of Rockland County and 12 surrounding counties found that 89 (8.3%) from 10 sewersheds tested positive for poliovirus type 2 (206).
Wastewater polio test results, by jurisdiction (N = 1,053)--13 counties in New York and New York City, March 9-October 11, 2022. Abbreviation: PV2 = poliovirus type 2. Sampling sites are sewersheds defined as the community area...
Sullivan (A), Orange (B), Rockland (C), Kings and Queens (D), and Nassau (E) counties, New York. Abbreviation: PV2 = poliovirus type 2. Sampling sites are sewersheds defined as the community area served by a wastewater collecti...
Modeling undetected poliovirus circulation following the 2022 outbreak in the United States demonstrated that in populations that maintain high overall immunization coverage with IPV: (1) rare polio cases may occur in un(der)-vaccinated individuals; and (2) type 2 outbreaks are possibly but unlikely to reestablish endemic transmission or result in large absolute numbers of paralytic cases (113; 232). Achieving and maintaining high immunization coverage with IPV is essential to prevent outbreaks and shorten the duration of imported poliovirus transmission (113). Suboptimal vaccine coverage in some areas and population groups is a significant challenge despite efforts to engage mothers, the key decision-makers on childhood vaccination (116).
Previous Advisory Committee on Immunization Practices (ACIP) recommendations for adult polio vaccination were limited to adults at increased risk for exposure; however, in 2023, ACIP recommended that all U.S. adults (aged ≥18 years) who are unvaccinated or incompletely vaccinated against polio complete a primary polio vaccination series with IPV (121).
WHO Eastern Mediterranean Region. Pakistan and Afghanistan are the only countries where endemic wild poliovirus transmission has never been interrupted and they are presently the only remaining countries with endemic WPV1 transmission (48; 05; 157; 154; 213; 71; 103; 101; 104; 149; 148; 150; 147; 50; 211; 234; 162; 194; 207; 137). During 2019 and 2020 (ie, durng the COVID-19 pandemic), these countries reported their highest numbers of WPV1 cases since 2014 and experienced outbreaks of type 2 circulating vaccine-derived poliovirus (cVDPV2) (154; 155; 162). There are major threats to increasing polio outbreaks due to the lack of accessibility to polio vaccines in these regions and neighboring areas (71; 149; 150; 147; 103).
Pakistan. The number of cases in Pakistan increased markedly from 2018 to 2019 (from 8 to 80 cases as of November 7 of each year, respectively) due to a breakdown of vaccinations resulting from vaccine refusals, chronically missed children, who remained unvaccinated, community campaign fatigue, and poor vaccination management and implementation (101). Pakistan’s polio eradication efforts suffered severe setbacks that began during 2018, with a sharp increase in wild poliovirus type 1 (WPV1) cases and positive environmental samples from systematic sewage sampling for WPV1 and circulating vaccine-derived poliovirus type (cVDPV2) in 2019 (104). The resurgence of WPV1 resulted from ongoing challenges reaching children in districts with endemic transmission and deterioration in the quality of supplementary immunization activities: supplementary immunization activities are mass national or subnational campaigns conducted for a brief period, from days to weeks, in which one dose of oral poliovirus vaccine is administered to all children aged less than 5 years, regardless of vaccination history. Efforts to halt transmission of WPV1 failed in 2019. During 2019, 147 WPV1 cases were reported in Pakistan, more than 12 times the 12 reported cases during 2018.
Abbreviations: cVDPV2, circulating vaccine-derived poliovirus type 2; WPV1, wild poliovirus type 1. (Source: Mbaeyi C, Baig S, Safdar RM, et al. Progress toward poliomyelitis eradication - Pakistan, January 2022-June 2023. MMWR...
Abbreviation: WPV1, wild poliovirus type 1. (Source: Mbaeyi C, Baig S, Safdar RM, et al. Progress toward poliomyelitis eradication - Pakistan, January 2022-June 2023. MMWR Morb Mortal Wkly Rep 2023;72[33]:880-5. Public domain.)...
From January to September 2020, 72 WPV1 cases were reported among 33 districts in four provinces, compared with 72 from 22 districts in four provinces during the same period in 2019. Since the detection of the first cVDPV2 case in July 2019 through September 15, 2020, 81 cases affecting 30 districts in six provinces were reported. With the emergence of cVDPV2, spread of both poliovirus types was exacerbated in 2020 by the social, healthcare, and economic disruption associated with the COVID-19 pandemic. The COVID-19 pandemic placed significant demands on the health care system and disrupted surveillance and vaccination activities, in part because leaders, laboratory personnel, and frontline workers previously responsible for polio surveillance and vaccination activities were reassigned to combat the escalating COVID-19 epidemic (208). Consequently, the National Emergency Operations Center suspended all polio supplementary immunization activities from March to July 2020.
After reporting a single WPV1 case in 2021, Pakistan reported 14 cases from April 1 to July 31, 2022 (154); all but one of the 14 WPV1 cases in Pakistan during 2022 were reported from North Waziristan district in Khyber Pakhtunkhwa.
Abbreviations: cVDPV2 = circulating vaccine-derived poliovirus type 2; WPV1 = wild poliovirus type 1. (Source: Mbaeyi C, Baig S, Safdar MR, et al. Progress toward poliomyelitis eradication--Pakistan, January 2021-July 2022. MMW...
Abbreviations: cVDPV2 = circulating vaccine-derived poliovirus type 2; WPV1 = wild poliovirus type 1. (Source: Mbaeyi C, Baig S, Safdar MR, et al. Progress toward poliomyelitis eradication--Pakistan, January 2021-July 2022. MMW...
An outbreak of cVDPV type 2 (cVDPV2), which began in Pakistan in 2019, was successfully contained with the last case in April 2021 (154). Despite program improvements, an estimated 400,000-500,000 children continue to be missed during nationwide polio supplementary immunization activities (154). Vaccination efforts were further complicated by months of flooding during the summer of 2022.
Afghanistan. The number of cases in Afghanistan has increased since 2017 (150; 147; 211; 207; 208). Insurgent groups banned house-to-house vaccination in effect from May to December 2018 in most southern and southeastern provinces, leaving approximately 1 million children inaccessible to oral poliovirus vaccine administration (150). From January to April 2019 vaccination was allowed at designated community sites, but since April 2019 vaccination campaigns were banned nationally in Afghanistan (150). During March through June 2020, all campaigns were paused because of the coronavirus disease 2019 (COVID-19) pandemic (147). The number of WPV1 cases reported in Afghanistan increased from 21 in 2018 to 29 in 2019. During January to July 2020, 41 wild poliovirus type 1 cases were reported as of August 29, 2020 (compared with 15 during the same period in 2019); in addition, 69 cases of circulating vaccine-derived poliovirus type 2 and 1 case of ambiguous vaccine-derived poliovirus type 2 (aVDPV2) were detected (147).
Poliovirus immunization had been compromised in Afghanistan by years of active conflict, which culminated with replacement of the Afghanistan government by the Taliban de facto government on August 15, 2021. In August and September of 2021, there was marked social and economic disruption in the country associated with the withdrawal of U.S. troops.
In Afghanistan, the number of WPV1 cases nearly doubled from 29 in 2019 to 56 in 2020; in addition, 308 cVDPV2 cases were reported during 2020 (162).
The number of cases of WPV1 and cVDPV2 were 90 and 351, respectively. cVDPVs are genetically linked VDPV2 isolates for which there is evidence of person-to-person transmission in the community. Abbreviations: cVDPV2 = circulati...
cVDPVs are genetically linked VDPV2 isolates for which there is evidence of person-to-person transmission in the community. Total cases by period: January-June 2021 = one WPV1 and 42 cVDPV2, July-December 2021 = three WPV1 and ...
Throughout 2021, four WPV1 and 43 cVDPV2 cases were detected, representing marked decreases from the prior year. From January to September 2022, two WPV1 cases (and no cVDPV2 cases) were detected. Although no supplementary immunization activities occurred from July to October 2021, supplementary immunization activities resumed in November 2021 in all districts after the political transition, and 3.5 to 4.5 million people have been vaccinated since.
Yemen. In 2009, Yemen had achieved polio-free status and maintained it until 2019, but ongoing political conflict since 2015, coupled with challenges in delivering the polio vaccine to conflict-affected areas, resulted in two polio outbreaks: 35 cases caused by VDPV1 from 2019 and 2021, and 230 cases caused by VDPV2 from November 2021 and throughout 2022 (10).
(Source: Al-Qassimi MA, Al Amad M, Al-Dar A, Al Sakaf E, Al Hadad A, Raja'a YA. Circulating vaccine-derived poliovirus type 2 outbreak and response in Yemen, 2021-2022, a retrospective descriptive analysis. BMC Infect Dis 2024;...
Abbreviation: cVDPV2, circulating vaccine-derived poliovirus type 2. (Source: Al-Qassimi MA, Al Amad M, Al-Dar A, Al Sakaf E, Al Hadad A, Raja'a YA. Circulating vaccine-derived poliovirus type 2 outbreak and response in Yemen, ...
Being affected by outbreaks of both cVDPV1 and cVDPV2 is an indicator of low polio vaccine coverage in Yemen (11; 10; 26).
WHO African Region. The Africa Regional Commission for the Certification of Poliomyelitis Eradication declared the African region polio-free of wild-type poliovirus in 2020 after meeting all the criteria for certification and after 3 years had passed without detection of any wild poliovirus in any country in the region. With the African region’s certification, five of the six WHO regions, representing over 90% of the world’s population, were declared free of wild polioviruses.
However, a 3-year-old girl developed poliomyelitis from WPV1 in November 2021 (143; 158; 193; 241; 244). The affected child had no history of travel or contact with anyone who had traveled internationally and had received only 1 of 5 recommended doses of poliovirus vaccine. Genomic sequence analysis of the isolated poliovirus indicated that its closest relative was a WPV1 lineage isolated in Sindh Province, Pakistan, in October 2019.
An additional case of paralytic WPV1 was reported in Tete Province, Mozambique, with paralysis onset in March 2022 (143).
In addition, outbreaks of VDPVs have developed in many African countries, jeopardizing polio eradication efforts (76; 153; 163; 06; 200).
Nigeria. Nigeria is among the countries with endemic poliomyelitis in recent years (47; 73; 72; 238; 176; 01; 20).
(Source: Bammeke P, Adamu US, Bolu O, et al. Descriptive epidemiology of poliomyelitis cases due to wild poliovirus type 1 and wild poliovirus type 3 in Nigeria, 2000-2020. Pan Afr Med J 2023;45[Suppl 2]:4. Creative Commons Att...
Cameroon. The last wild poliovirus was reported in Cameroon in 2014, and no cases of poliovirus were detected in the country from 2015 to 2018. After that, an increasing number of cVDPV2 were reported from 2019 to 2022 (175; 174).
The last confirmed case due to wild poliovirus type 1 in Nigeria was in August 2016 (38; 03). However, since 2018, cVDPV2 has emerged and spread from Nigeria to Niger and Cameroon (175; 174); outbreak responses have so far not interrupted transmission (03).
Angola. After 6 years without any detection of poliomyelitis cases, Angola experienced an outbreak of cVDPV2 beginning in 2019, with 141 cVDPV2 polio cases reported from all 18 provinces from 2019 to 2020 (165). These cases were classified into five distinct genetic emergence groups with overlap to cases identified in 2017 to 2018 in the Democratic Republic of Congo. From June 2019 to July 2020, supplementary immunization campaigns were conducted using monovalent oral poliovirus vaccine type 2 (mOPV2) that successfully interrupted transmission of cVDPV2 early in 2020.
South Sudan. The emergence of the circulating vaccine-derived poliovirus type 2 outbreak in South Sudan in 2020–2021 was due to low population immunity (226). Between September 2020 and April 2021, 59 cases of the circulating virus were confirmed in the country, with 50 cases in 2020 and nine cases in 2021.
Abbreviations: cVDPV, circulating vaccine-derived poliovirus; mOPV2, monovalent oral polio vaccine type 2; NID, national immunization day; sNID, supplemental national immunization day. (Source: Tegegne AA, Anyuon AN, Legge GA, ...
More cases were males (56%) under five (93%). The immunization histories of confirmed cases indicated that 24% never received any doses of oral polio or injectable vaccines either from routine or during supplemental immunization before the onset of paralysis, 29% received 1 to 2 doses, and 48% received three or more doses. In response to the outbreak, two national immunization campaigns and a mop-up were conducted with monovalent oral polio vaccine type 2 (mOPV2), with administrative coverage of 91%, 99%, and 97% for the first, second, and mop-up rounds, respectively.
WHO European Region: free of wild-type poliovirus (2002). The last wild poliovirus infection in Europe was in 1998, and the WHO declared the European Region polio-free of wild-type poliovirus in 2002. Since 2002, the European Region has experienced several importations of either wild poliovirus or VDPV, requiring comprehensive response efforts.
United Kingdom. In early 2022, VDPV2 was detected in environmental samples in London, United Kingdom; no associated cases of paralysis were detected, and the technical definition and criteria for "circulation" of VDPV2 were not met. The British government immediately reinstated polio vaccination for all children (151). Recent coverage in London for the primary course of DTaP/IPV/Hib/HepB vaccination, which includes protection against polio, suggests immunization coverage of 87%.
Israel. In September 2021, increasing numbers of wastewater samples collected from more than one site in Jerusalem, Israel, tested positive for ambiguous type 3 vaccine-derived poliovirus (aVDPV3), whereas environmental samples from other sampling sites were negative (239). In late February 2022, a VDPV3, genetically related to the Jerusalem environmental surveillance samples, was isolated from a stool sample collected from a non-immunodeficient, non-immunized child from Jerusalem who developed paralytic poliomyelitis, indicating that the aVDPV3s were circulating (cVDPV3s) rather than immunodeficiency-related VDPV3s (iVDPVs). In response, the Israel Ministry of Health launched a catch-up immunization program.
Sweden. Since the introduction of polio vaccination in Sweden, an estimated 58,240 cases of polio have been averted, although this is likely a significant underestimate due to underreporting during pre-vaccination years (146).
Observed incidence and median (95% CI) pre-vaccination incidence based on the main and sensitivity analyses, Sweden 1910- to 2019. Gray-shaded areas represent pre-vaccination periods. CI, confidence interval. (Source: Martin LJ...
WHO South-East Asia Region: free of wild-type poliovirus (2014). The WHO South-East Asia Region was certified polio-free of wild-type poliovirus in 2014.
WHO Western Pacific Region: free of wild-type poliovirus (2000). The WHO Western Pacific Region was certified polio-free of wild-type poliovirus in 2000.
Papua New Guinea. Since June 2018 poliovirus is again circulating in Papua New Guinea after that country was polio-free for 18 years (92); the current outbreak is caused by vaccine-derived poliovirus, an indicator of low polio vaccine coverage in the country.
|
• Both the inactivated poliomyelitis vaccine (the Salk vaccine) and the live, attenuated oral poliovirus vaccine (the Sabin vaccine) have effectively decreased the incidence of poliomyelitis. | |
|
• A person is considered fully immunized if he or she has received a primary series of at least three doses of inactivated poliovirus vaccine, live oral poliovirus vaccine, or four doses of any combination of inactivated poliovirus vaccine and oral poliovirus vaccine. | |
|
• To eliminate the risk of vaccine-associated paralytic poliomyelitis, as of 2000, oral poliovirus vaccine was no longer recommended for routine immunization in the United States. | |
|
• The World Health Organization plans to withdraw all oral polio vaccines and switch to the sole use of inactivated poliovirus vaccine. However, this will only occur after global eradication of wild poliovirus has been certified, which will be at least 3 years after the last case of wild poliovirus infection. | |
|
• Because poliovirus type 2 has been eradicated worldwide, the type 2 component of the oral poliovirus vaccine is targeted for global withdrawal through a switch from the trivalent oral poliovirus vaccine to a bivalent oral poliovirus vaccine. This change is intended to prevent paralytic polio caused by circulating vaccine-derived poliovirus type 2. |
Vaccination has been essential in the fight to eradicate polio because the virus can only be transmitted from person to person, and vaccination breaks this cycle of infection. Both the inactivated poliomyelitis vaccine (the Salk vaccine) and the live, attenuated oral poliovirus vaccine (the Sabin vaccine) have been effective in decreasing the incidence of poliomyelitis (186; 42). Today, a person is considered fully immunized if he or she has received a primary series of at least three doses of inactivated poliovirus vaccine, live oral poliovirus vaccine, or four doses of any combination of inactivated poliovirus vaccine and oral poliovirus vaccine.
Previously, the benefits of oral poliovirus vaccine use (ie, intestinal immunity, secondary spread) outweighed the risk for vaccine-associated paralytic poliomyelitis (ie, an adverse event following exposure to oral poliovirus vaccine), which occurred in one child out of every 2.4 to 2.5 million oral poliovirus vaccine doses distributed (247).
To eliminate the risk of vaccine-associated paralytic poliomyelitis, as of 2000, oral poliovirus vaccine was no longer recommended for routine immunization in the United States. Russia used oral polio vaccine from 1998 to 2007 and inactivated polio vaccine followed by oral polio vaccine from 2008 to 2014 (107). China began introducing inactivated polio vaccine at the end of 2014, and so far, it has been successful (250). However, oral poliovirus vaccine continues to be used in the countries where polio is either endemic or the risk of importation and transmission is high. Prevention can also occur by stopping the spread of infection using good personal, family, and public hygiene.
The World Health Organization plans to withdraw all oral polio vaccines and switch to the sole use of inactivated poliovirus vaccine (187). However, this will only occur after global eradication of wild poliovirus has been certified, which will be at least 3 years after the last case of wild poliovirus infection (187).
Because wild-type poliovirus type 2 has been eradicated worldwide, the type 2 component of the oral poliovirus vaccine is targeted for global withdrawal through a switch from the trivalent oral poliovirus vaccine to a bivalent oral poliovirus vaccine (192). This change is intended to prevent paralytic polio caused by circulating vaccine-derived poliovirus type 2.
Changes in polio vaccines after eradication of wild poliovirus type 2. On September 20, 2015, the Global Commission for the Certification of Eradication declared that wild poliovirus type 2 has been eradicated worldwide. With that declaration, it was necessary to develop a new immunization regimen that solely addresses serotypes 1 and 3 using oral polio vaccine (to minimize the risk of vaccine-derived cases of type 2) (169; 223). As of May 1, 2016, the use of oral poliovirus vaccine type 2 for routine and supplementary immunization ceased after a synchronized global switch from trivalent oral poliovirus vaccine (tOPV), containing Sabin strain types 1, 2, and 3, to bivalent oral poliovirus vaccine (bOPV), containing Sabin strain types 1 and 3 (08).
Since the global switch in poliovirus vaccines in 2016, monovalent oral poliovirus vaccine type 2 (mOPV2), containing Sabin strain type 2, has been used for cVDPV type 2 outbreaks; tOPV continues to be available if cVDPV2 co-circulates with wild poliovirus type 1, and bOPV is used for outbreaks of cVDPV type 1 or type 3.
A genetically stable novel type 2 oral poliovirus vaccine (nOPV2), produced by genetic modification of the type 2 Sabin vaccine virus genome, was developed and evaluated through phase I and phase II clinical trials from 2017 to 2019 (145). nOPV2 was demonstrated to be safe and well-tolerated, have noninferior immunogenicity, and have superior genetic stability compared with the Sabin monovalent type 2 vaccine (08; 145; 21). The World Health Organization authorized nOPV2 for use under the Emergency Use Listing pathway in November 2020, allowing its first use for outbreak response in March 2021 (145; 21). Subsequent global nOPV2 genomic surveillance from March to October 2021 confirmed the genetic stability of the primary attenuating site (145). In October 2021, the WHO's principal advisory group permitted wider use of nOPV2, but the current nOPV2 supply is limited.
A clinical diagnosis of paralytic poliomyelitis is usually straightforward in countries where polio has not been eradicated. A physician should have a high index of suspicion of polio in any patient presenting with acute-onset fever, nuchal rigidity, and asymmetrical flaccid muscle paresis with hyperesthesia and relative preservation of somatosensation. Absent or poor immunization history against poliomyelitis will also strengthen the clinical suspicion. Identification of poliovirus in stool or CSF confirms the diagnosis.
Other causes of acute flaccid paralysis include Guillain-Barre syndrome and acute transverse myelitis. Although polio is not as common as Guillain-Barre syndrome or acute transverse myelitis, the importance of correct diagnosis cannot be overemphasized due to its high risk of transmission (79).
Guillain-Barre syndrome is a leading cause of acute flaccid paralysis and presents as a rapidly progressive, symmetric flaccid paralysis; there may also be a distal paresthesia with CSF cytoalbuminologic dissociation early in the disease. Although myalgias and fever at onset of paralysis are prominent features in paralytic polio, they are not as common during Guillain-Barre syndrome (79).
Flaccid paralysis may also occur in the early phase of acute transverse myelitis; however, accompanying sensory symptoms (eg, anesthesia, paresthesia with a sensory level) and neurogenic bowel and bladder dysfunction differentiate it from polio (79).
During acute flaccid paralysis surveillance in Australia from 2007 to 2017, the annual incidence of acute flaccid paralysis was 1.3 cases per 100,000 children aged younger than 15 years with Guillain-Barre syndrome (36%), transverse myelitis (17%), and acute disseminated encephalomyelitis (15%) the major causes identified (22). Periods of increased acute flaccid paralysis frequency in 2013 and 2016 coincided with increased reporting of non-polio anterior horn cell disease and detection of non-polio enterovirus.
In recent years, West Nile virus has increased in geographical spread. Although the neurologic manifestations of West Nile virus typically include meningitis and encephalitis, acute flaccid paralysis may also occur, ranging from monoparesis to quadriparesis, with or without bulbar symptoms. Acute flaccid paralysis due to West Nile virus can be clinically difficult to differentiate from poliomyelitis. A history of mosquito bites, a “summer flu,” and acute flaccid paralysis may suggest West Nile virus in endemic areas.
Diphtheric polyneuropathy is a vaccine-preventable bacterial infection that can also produce fever and acute flaccid paralysis (commonly symmetric) and should be considered in the differential diagnosis of patients presenting with acute flaccid paralysis in countries where poliomyelitis and diphtheria have not been eradicated (152).
Other viral respiratory or gastrointestinal illnesses can present with features similar to minor nonparalytic poliomyelitis (79). In addition, other non-polio enteroviruses have been associated with acute flaccid paralysis (136; 97; 28). An acute flaccid paralysis surveillance report in India found Coxsackie and Echovirus the most common non-polio enterovirus isolates implicated (136). In 2014, the United States experienced a nationwide outbreak of enterovirus D68 (EV-D68) in which a total of 1152 people, particularly children, in 49 states and the District of Columbia, were confirmed with respiratory illness.
During the outbreak, the Centers for Disease Control and Prevention was notified by the Colorado Department of Public Health and Environment of a cluster of nine children evaluated at Children's Hospital Colorado with acute neurologic illness characterized by extremity weakness, cranial nerve dysfunction (eg, diplopia, facial droop, dysphagia, or dysarthria), or both (184). Enterovirus D68 has since been recognized as a cause of polio-like illness (19; 43; 128; 144; 160; 55; 210; 243). Isolated cases of poliomyelitis-like illness have been reported elsewhere since the 2014 United States outbreak (221).
The paralytic presentation of rabies virus infection (so-called “dumb” rabies) may also be confused with poliomyelitis. A history of an animal bite should warrant consideration of rabies (85). Outbreaks of dumb rabies have occurred in humans as a result of bites from vampire bats (129; 130).
Other conditions that may give a polio-like picture include spinal cord compression; tick, snake, or spider bite; botulism; and toxic substances such as arsenic (82; 19).
|
• Viral isolation, serology, and polymerase chain reaction assays can be used to confirm the laboratory diagnosis of poliomyelitis. | |
|
• A laboratory diagnosis of poliomyelitis is made by isolating the virus from stool or pharynx. Virus isolation is highest in stool, lower in the pharynx, and lowest in blood and CSF. | |
|
• The use of serological tests in the diagnosis of polio depends on the rise in antibody titer between the acute and convalescent phases; a serum specimen should be obtained as early as possible during the acute phase as well as 4 weeks later (convalescent phase). A 4-fold rise in titer between the acute and convalescent phase specimens suggests infection. | |
|
• CSF abnormalities are present in up to 90% of poliomyelitis cases. | |
|
• Post-polio syndrome is a diagnosis of exclusion based on evidence of new muscle weakness or muscle fatigability that is persistent for at least 1 year. | |
|
• Electromyographic and muscle biopsy findings, including evidence of ongoing denervation, cannot reliably distinguish between patients with or without post-polio syndrome. |
Following a clinical suspicion of polio, confirming the diagnosis and reporting the case to the local and national public health department is imperative. Early detection will allow timely measures, such as successful vaccination, to limit the spread and promote poliomyelitis eradication. Viral isolation, serology, and polymerase chain reaction assays can be used to confirm the laboratory diagnosis of poliomyelitis.
A laboratory diagnosis of poliomyelitis is made by isolating the virus from stool or pharynx. Virus isolation is highest in stool, lower in the pharynx, and lowest in blood and CSF. CSF viral isolation, though rare, is diagnostic (237). It is recommended that at least two stool specimens and two throat swabs be obtained 24 hours apart from suspected poliomyelitis patients as early as possible (237). The frequency of obtaining samples increases the likelihood of viral isolation as there is intermittent shedding of the virus from the mucous membranes. Although poliovirus has been the most common enterovirus isolated in the stool in cases of acute flaccid paralysis, a small proportion of non-poliovirus infections can also be isolated (02).
The use of serological tests in the diagnosis of polio depends on the rise in antibody titer between the acute and convalescent phases; a serum specimen should be obtained as early as possible during the acute phase as well as 4 weeks later (convalescent phase). A 4-fold rise in titer between the acute and convalescent phase specimens suggests infection (237). False negatives can occur in the immunocompromised. Serology cannot differentiate wild virus from antibodies generated from vaccination. Serology is used to detect the degree of protection conferred by polio vaccination (220). Polymerase chain reaction can be used to differentiate between wild and vaccine types of poliovirus as well as the various strains of poliovirus (02).
CSF abnormalities are present in up to 90% of poliomyelitis cases. Typically, the CSF white blood cell count ranges from 10 to 200 cells/µl. A polymorphonuclear leukocytosis is followed by a mononuclear response. CSF protein is typically normal or mildly elevated (79).
Post-polio syndrome is a diagnosis of exclusion that is made based on evidence of new muscle weakness or muscle fatigability that is persistent for at least 1 year (139). Electromyographic and muscle biopsy findings, including evidence of ongoing denervation, cannot reliably distinguish between patients with or without post-polio syndrome (139).
|
• Initial treatment of paralytic poliomyelitis relies on supportive care followed by passive and then active physical therapy and orthopedic measures. During the preparalytic stage, which would only be suspected during epidemics, bed rest is recommended because physical exercise can increase the severity of paralysis. | |
|
• Paralytic disease in the acute stage should be treated similarly the preparalytic stage with the following additions: (1) hospitalization is required; (2) respiratory support on a ventilator may be required; and (3) appropriate positioning such as splints to prevent contractures, foot boards to prevent foot drop, and frequent turning to prevent decubiti should be provided. |
Initial treatment of paralytic poliomyelitis relies on supportive care followed by passive and then active physical therapy and orthopedic measures.
During the preparalytic stage, which would only be suspected during epidemics, bed rest is recommended because physical exercise can increase the severity of paralysis (111). In addition, sore muscles can be treated with hot packs, fever with acetaminophen, and anxiety with anxiolytics. Paralytic disease in the acute stage should be treated in a similar fashion as the pre-paralytic stage with the following additions: (1) hospitalization is required; (2) respiratory support on a ventilator may be required; and (3) appropriate positioning such as splints to prevent contractures, foot boards to prevent foot drop, and frequent turning to prevent decubiti should be provided (191). Patients with bulbar involvement may require fluid and electrolyte support and additional support if cardiovascular, respiratory, or autonomic dysfunction occurs (236). Physical therapy is deferred until the convalescent stage, except for gentle passive range of motion to prevent contractures. During the convalescent period, active physical therapy with non-fatiguing muscle-strengthening exercises and hydrotherapy can be instituted. Patients with late and new effects of polio may need individualized multidisciplinary intervention and physiotherapy (25; 139; 140). Interventions may include adaptive equipment, orthotic equipment, gait/mobility aids, and a variety of therapeutic exercises (140). A meta-analysis of 21 studies showed that muscle strengthening and cardiovascular interventions among polio survivors are helpful in improving outcomes according to the International Classification of Functioning, Disability, and Health (195).
Orthopedic surgical intervention (eg, arthrodesis, tendon transfers, leg-shortening measures) should be deferred for 1.5 to 2 years after maximum recovery has been obtained (196; 108). For patients with residual effects of poliomyelitis, surgeons should optimize the position, choice, approach, and soft tissue tensioning because stability of prosthesis may be difficult to ensure and maintain (125).
Pregnancy increases the incidence of paralytic disease by about 3-fold (199). It is unclear if this increased risk is related to hormonal changes or a greater exposure to small children (218).
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Douglas J Lanska MD MS MSPH
Dr. Lanska of the University of Wisconsin School of Medicine and Public Health and the Medical College of Wisconsin has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Infectious Disorders
Oct. 08, 2024
Infectious Disorders
Sep. 13, 2024
Infectious Disorders
Aug. 27, 2024
Infectious Disorders
Aug. 27, 2024
Infectious Disorders
Aug. 26, 2024
General Neurology
Aug. 14, 2024
Neuromuscular Disorders
Aug. 11, 2024
Neurobehavioral & Cognitive Disorders
Aug. 11, 2024